Disclosure Information Devices for ASDPFO Closure Amplatzer Devices







- Slides: 15

Disclosure Information Devices for ASD&PFO Closure: Amplatzer Devices Alpay Celiker M. D. As a faculty member for this program, I disclose the following relationships with industry: No Conflict Of Interest

ASD/PFO Closure Amplatzer Devices Alpay Celiker M. D. Acibadem University, Department of Pediatric Cardiology Istanbul, Turkey.

Amplatzer Devices Used for ASD/PFO Closures • Amplatzer Septal Occluder • Cribriform Occluder • Amplatzer PFO Occluder

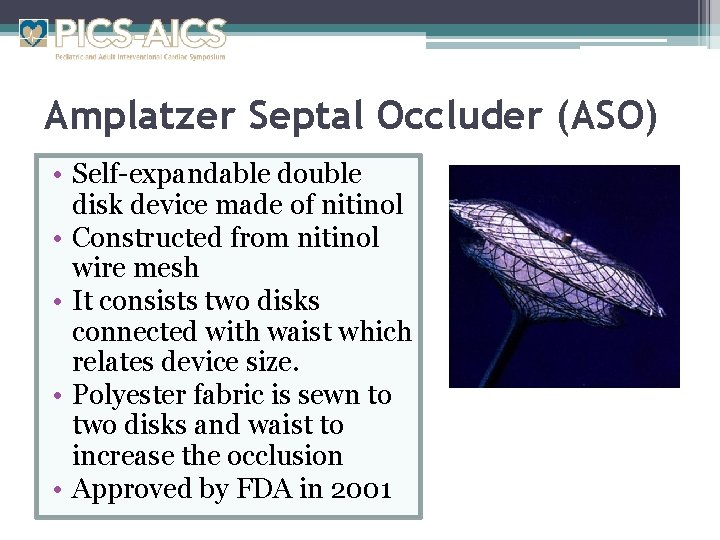
Amplatzer Septal Occluder (ASO) • Self-expandable double disk device made of nitinol • Constructed from nitinol wire mesh • It consists two disks connected with waist which relates device size. • Polyester fabric is sewn to two disks and waist to increase the occlusion • Approved by FDA in 2001

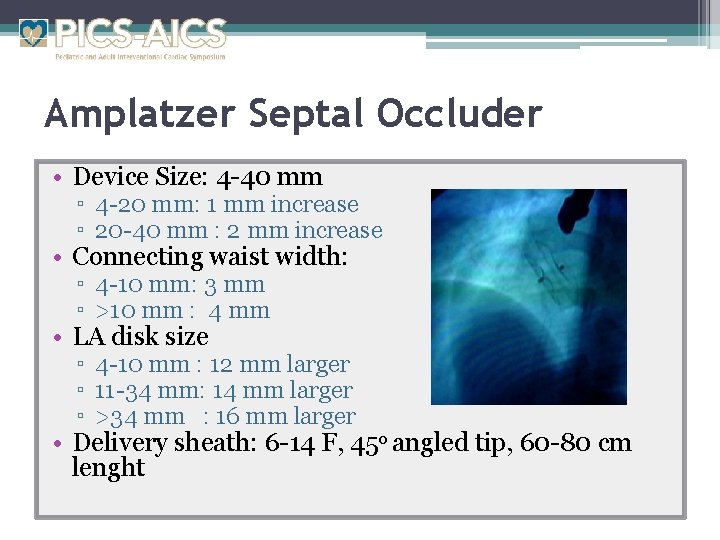
Amplatzer Septal Occluder • Device Size: 4 -40 mm ▫ 4 -20 mm: 1 mm increase ▫ 20 -40 mm : 2 mm increase • Connecting waist width: ▫ 4 -10 mm: 3 mm ▫ >10 mm : 4 mm • LA disk size ▫ 4 -10 mm : 12 mm larger ▫ 11 -34 mm: 14 mm larger ▫ >34 mm : 16 mm larger • Delivery sheath: 6 -14 F, 45 o angled tip, 60 -80 cm lenght

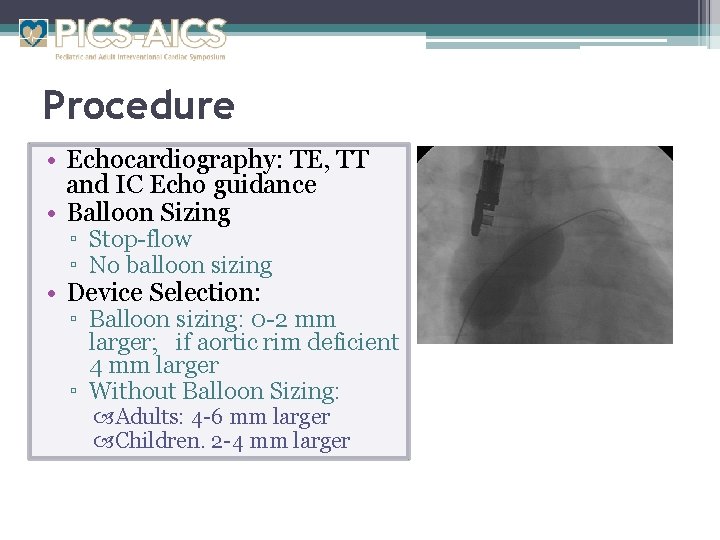
Procedure • Echocardiography: TE, TT and IC Echo guidance • Balloon Sizing ▫ Stop-flow ▫ No balloon sizing • Device Selection: ▫ Balloon sizing: 0 -2 mm larger; if aortic rim deficient 4 mm larger ▫ Without Balloon Sizing: Adults: 4 -6 mm larger Children. 2 -4 mm larger

ASO: Results (FDA DATA) ▫ Procedure success rate 97, 6 % ▫ Complete closure rate: 1 day: 96, 7 %, 6 months: 97, 2 %, 1 year: 98, 5 % ▫ Major adverse events: 7 (1, 6 %) Device embolization: 4, Cardiac arrhythmia: 2, Delivery system failure: 1 ▫ Minor adverse events: 27 (6, 1 %) Cardiac arrhythmia: 15, Thrombus formation: 3, Headache, allergic reaction and delivery system failure: 6

ASO: Complications • Device Embolization/Migration: % 1 • Arrhythmia: Supraventricular arrhythmias are more common at immediate period. • Cardiac Erosion and Perforation: Reported as 0, 1 % of patients. Cardiac perforations involve the anterio-superior atrial wall or adjacent aorta. It may be seen in patients with deficient aortic and posterior rim with use of oversized defect. High-risk patients should be followed by serial echo exams in patients with increasing or new pericardial effusion at the next day echo. • Thrombus Formation • Cobra-head Formation

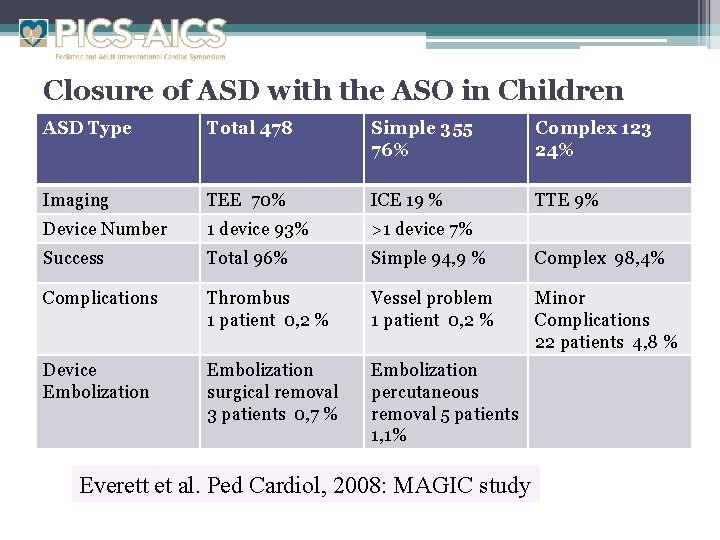
Closure of ASD with the ASO in Children ASD Type Total 478 Simple 355 76% Complex 123 24% Imaging TEE 70% ICE 19 % TTE 9% Device Number 1 device 93% >1 device 7% Success Total 96% Simple 94, 9 % Complex 98, 4% Complications Thrombus 1 patient 0, 2 % Vessel problem 1 patient 0, 2 % Minor Complications 22 patients 4, 8 % Device Embolization surgical removal 3 patients 0, 7 % Embolization percutaneous removal 5 patients 1, 1% Everett et al. Ped Cardiol, 2008: MAGIC study

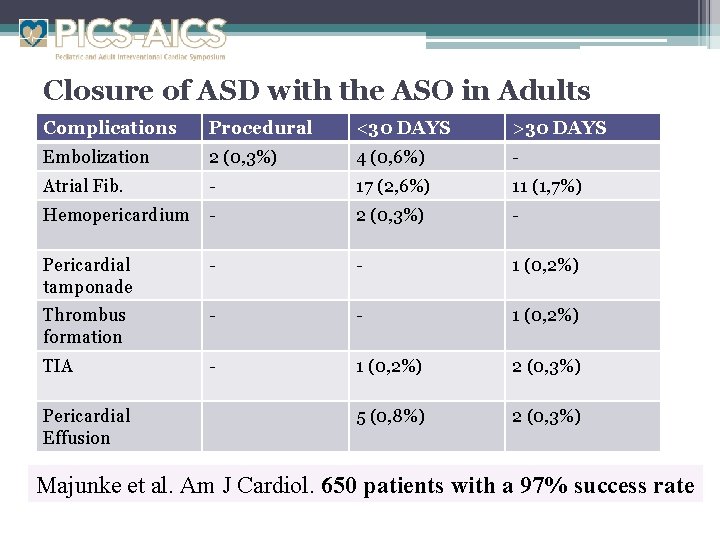
Closure of ASD with the ASO in Adults Complications Procedural <30 DAYS >30 DAYS Embolization 2 (0, 3%) 4 (0, 6%) - Atrial Fib. - 17 (2, 6%) 11 (1, 7%) Hemopericardium - 2 (0, 3%) - Pericardial tamponade - - 1 (0, 2%) Thrombus formation - - 1 (0, 2%) TIA - 1 (0, 2%) 2 (0, 3%) 5 (0, 8%) 2 (0, 3%) Pericardial Effusion Majunke et al. Am J Cardiol. 650 patients with a 97% success rate

Cribriform Occluder • Designed to closure multifenestrated defect • Both disk diameters are equal and connecting waist is short. • Disk sizes ▫ 18 mm , 25 mm, 30 mm, 35 mm, 40 mm (not available in US)

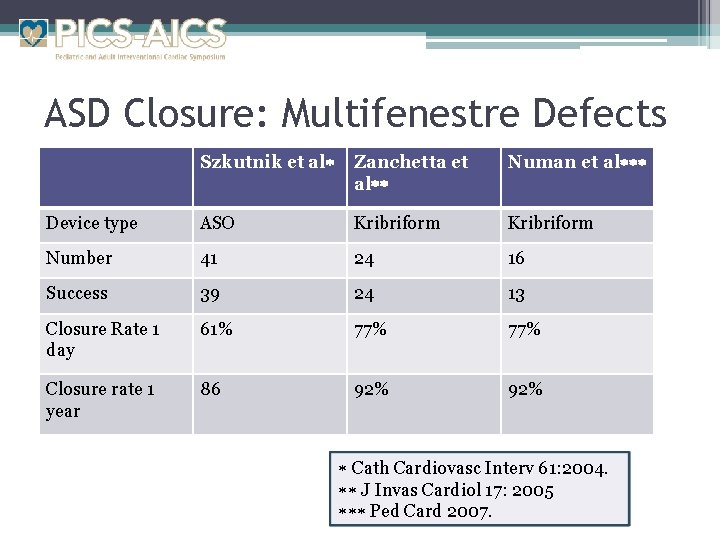
ASD Closure: Multifenestre Defects Szkutnik et al Zanchetta et al Numan et al Device type ASO Kribriform Number 41 24 16 Success 39 24 13 Closure Rate 1 day 61% 77% Closure rate 1 year 86 92% Cath Cardiovasc Interv 61: 2004. J Invas Cardiol 17: 2005 Ped Card 2007.

Amplatzer PFO Occluders Three sizes • 18 mm : Both disks are 18 mm • 25 mm: Right disc 25 mm, left disc 18 mm • 35 mm: Right disc 35 mm, left disc 28 mm

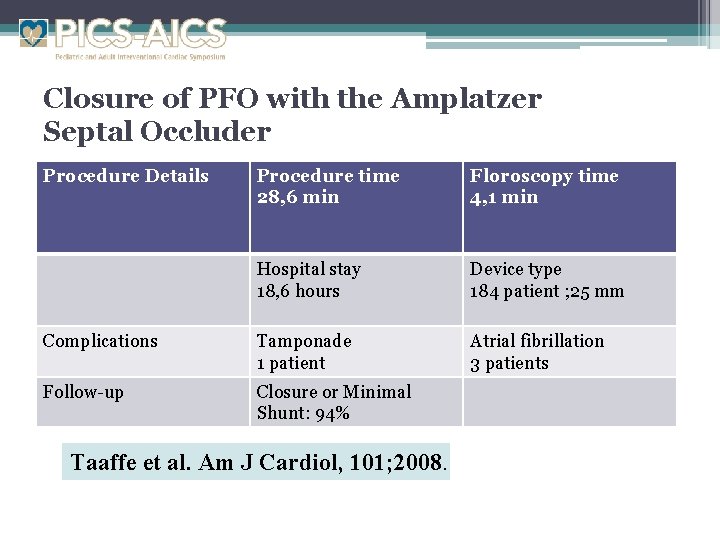
Closure of PFO with the Amplatzer Septal Occluder Procedure Details Procedure time 28, 6 min Floroscopy time 4, 1 min Hospital stay 18, 6 hours Device type 184 patient ; 25 mm Complications Tamponade 1 patient Atrial fibrillation 3 patients Follow-up Closure or Minimal Shunt: 94% Taaffe et al. Am J Cardiol, 101; 2008.

Thank you