Use of SAS for Clinical Trial Management and








- Slides: 27

Use of SAS® for Clinical Trial Management and Risk-Based Monitoring of Multicenter Clinical Trial Data from Electronic Data Capture Tools Bob Hall 1, MS, Rebecca V. Fink 2 MPH, David Gagnon 1, MD, MPH, Ph. D NESUG 2013 Presentation

Objectives of Presentation: § Introduce Risk-Based Monitoring and Electronic Data Capture § Discuss Business Use Cases § Provide a SAS Approach for Generating Modular Reports for Site Monitoring Metrics

Multi-Center Clinical Trials: § Multi-center trials can be complicated: • Multiple visits for long term trials • Multiple CRFs with different purposes ØSafety (e. g. Adverse Events, Pharmacy Data) ØEfficacy (e. g. Outcomes Data for Trial Objectives) ØStudy Activity (e. g. Protocol Deviations; Disposition) § Trials require frequent monitoring of data to insure quality.

Risk-Based Monitoring: § Trial monitoring has focused on on-site activities § FDA Guidance Document that describes a riskbased approach to monitoring: • Recently finalized – August 2013 • Combines on-site monitoring and remote ‘centralized monitoring’. • Targeted monitoring of site activity remotely.

Risk-Based Monitoring: § Improve efficiency and costs of clinical trials • Reducing the need for frequent on-site monitoring. • Focus on sites that don’t meet defined study metrics. • Dictate corrective actions with sites: Ø Initiate re-training efforts, improve communication with sites, increase on-site monitoring activities. Ø Probation efforts if needed.

Electronic Data Capture: § Electronic Data Capture (e. DC): • Real-time data capture and correction. • Data maintained in relational database architecture. • Trial management functions (CRFs, DCFs, etc. ). § Operational Tables for Trial Management: • • Participant Status CRF/Form Status DCF Status Master Data Files (i. e. tables for all data transactions)

SAS for Reporting: § Points to Consider - SAS for Risk-Based Monitoring • Output Delivery System (ODS) Functionality Ø Ability to establish professional level reports in many formats. • Physical Report Document Ø Found sites were more responsive to a physical report. • Reports in Near Real Time Ø Daily reports based on schedule cron jobs. Ø Timing was considered acceptable for task. • SAS Knowledge Base Ø Research infrastructure had more SAS experience.

Business Use Cases: Business Use Cases for Discussion: 1) Case Report Form (CRF) Completion 2) Data Clarification (DCF) Status

Case Report Form (CRF) Completion: § Business Use Case: • Important to have data entered during or soon after participant visits. • Report for Site Staff: Ø Listing of outstanding CRFs Ø Days outstanding from expected Ø Summary numbers • Report for Management Teams: Ø CRF completion rates by site Ø Completion rates by CRF and visit window

CRF Completion: § Form Status Table • Captured information on form status markers. • Record for each expected CRF during course of study. § Determination of Incomplete CRFs Based on: • Date of participant enrollment • Expected date of study visit • Acceptable grace period for completion

CRF Completion:

CRF Completion: § Points to Consider: • Timing of Report Generation Ø Report frequency Ø Ability to generate ad hoc reports • Time Limit / Grace Period for CRF Completion Ø Protocol defined / May vary by CRF type • Risk-Based Monitoring Ø Overall metrics: ²Insure that percentage of CRFs complete w/in time limit v. No more than 10% outside 14 days ²Graphing completion rates from site launch

Monitoring Data Clarifications: § Flow of Data Clarification (DCF) : • DCFs Types: Ø Automated – Fire through a system script Ø Manual – Entered by coordinating center • Expected Site Responses: Ø Data correction because of true data error. Ø Resolving DCF since data value is correct. • Good Metric for Assessing DCF Correction is DCF Aging.

Monitoring Data Clarifications: § Business Use Case: • e. DC system - Didn’t Provide Best Metric for DCF Aging: Ø Based on date/time DCF opened by staff. Ø Aging continued after site response. • Build a Better Report: Ø Use DCF status table Ø Re-calculate aging based on time DCF fired Ø Define calculation solely on site action Ø Develop reports for sites and management teams

SAS Tools for Reporting: § SAS Tools for Reporting: • Output Delivery System (ODS) Functionality Ø Professional level reports in many formats (HTML, PDF, RTF) • Access to SQL Relational Databases Ø ODBC Connections • Macros to Facilitate Reporting Ø Macro Looping to Generate by Site Reports Ø Customized Reporting of Study Benchmarks

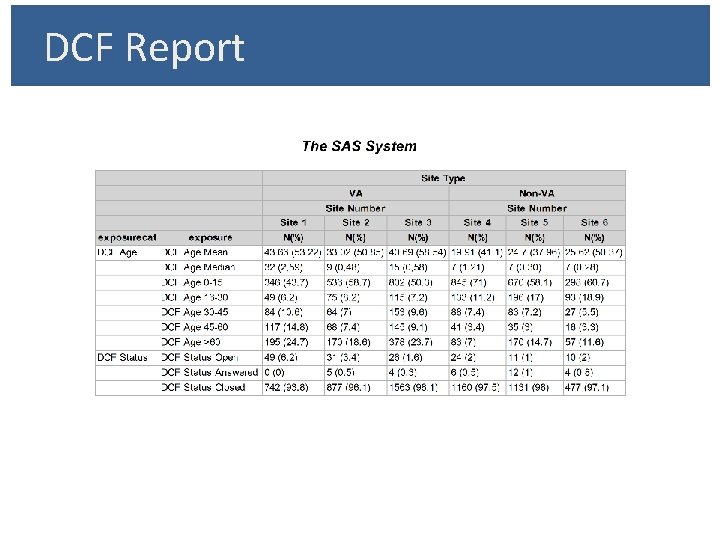
Customized DCF Report: § Business Case • Aggregate Report of Site Level Activity Ø Example - DCF Aging • Repeatability of Reports • Professional Looking Report – ODS Approach • Modular Reporting Process Ø Flexibility in Defining/Re-Defining Metrics Ø Flexibility in Ordering of Metrics in Report

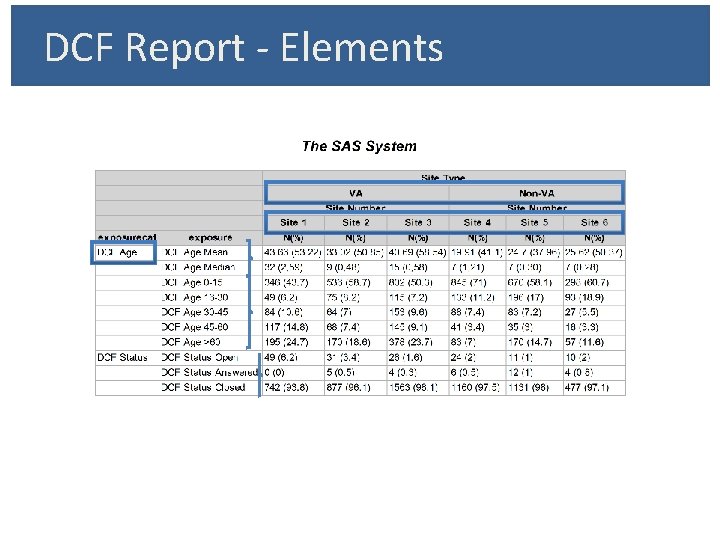
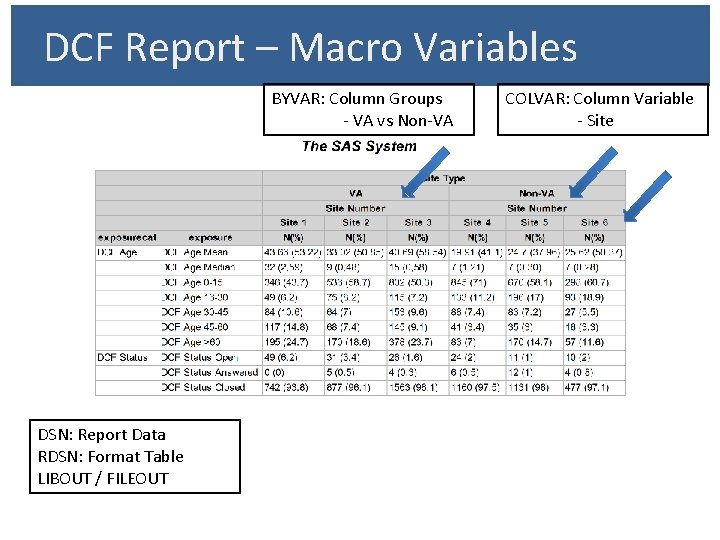
DCF Report - Elements

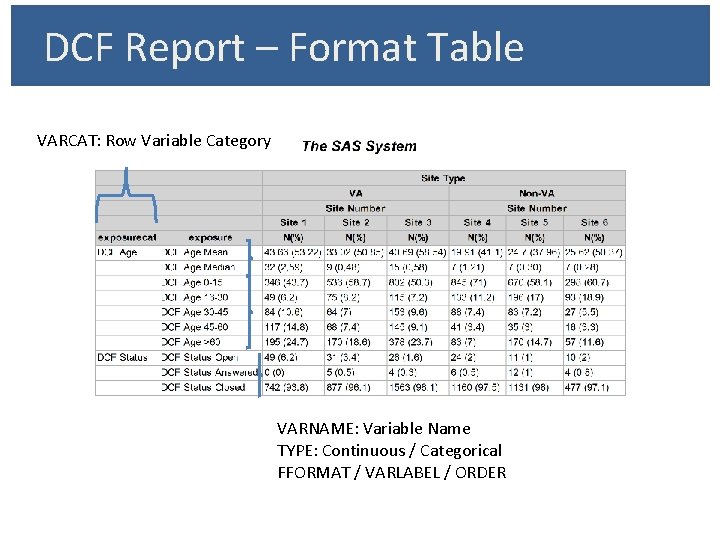
DCF Report – Format Table VARCAT: Row Variable Category VARNAME: Variable Name TYPE: Continuous / Categorical FFORMAT / VARLABEL / ORDER

DCF Report – Macro Variables BYVAR: Column Groups - VA vs Non-VA DSN: Report Data RDSN: Format Table LIBOUT / FILEOUT COLVAR: Column Variable - Site

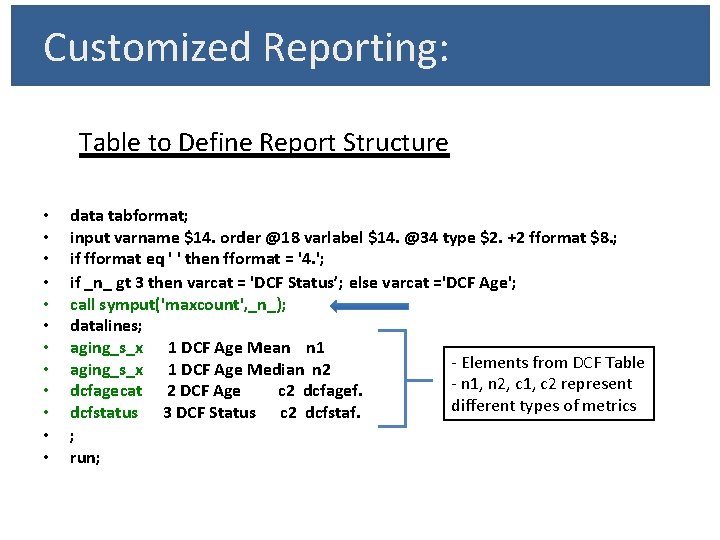
Customized Reporting: Table to Define Report Structure • • • data tabformat; input varname $14. order @18 varlabel $14. @34 type $2. +2 fformat $8. ; if fformat eq ' ' then fformat = '4. '; if _n_ gt 3 then varcat = 'DCF Status’; else varcat ='DCF Age'; call symput('maxcount', _n_); datalines; aging_s_x 1 DCF Age Mean n 1 - Elements from DCF Table aging_s_x 1 DCF Age Median n 2 - n 1, n 2, c 1, c 2 represent dcfagecat 2 DCF Age c 2 dcfagef. different types of metrics dcfstatus 3 DCF Status c 2 dcfstaf. ; run;

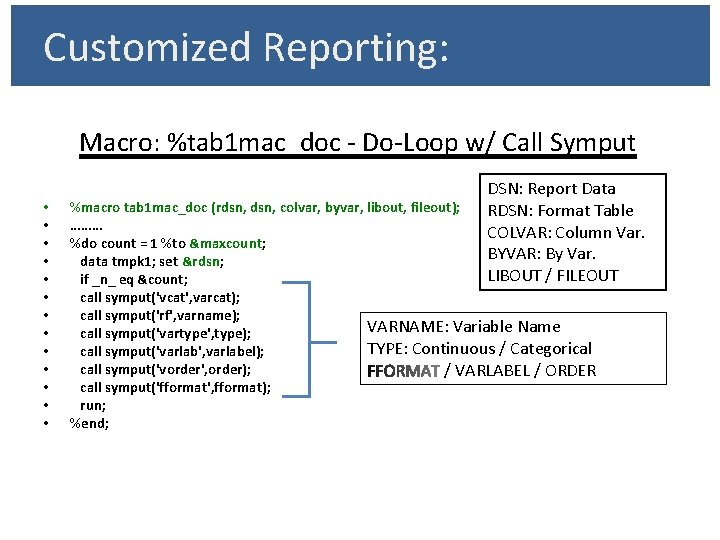
Customized Reporting: Macro: %tab 1 mac_doc - Do-Loop w/ Call Symput • • • • DSN: Report Data RDSN: Format Table COLVAR: Column Var. BYVAR: By Var. LIBOUT / FILEOUT %macro tab 1 mac_doc (rdsn, colvar, byvar, libout, fileout); ……… %do count = 1 %to &maxcount; data tmpk 1; set &rdsn; if _n_ eq &count; call symput('vcat', varcat); call symput('rf', varname); VARNAME: Variable Name call symput('vartype', type); TYPE: Continuous / Categorical call symput('varlab', varlabel); call symput('vorder', order); / VARLABEL / ORDER call symput('fformat', fformat); run; %end;

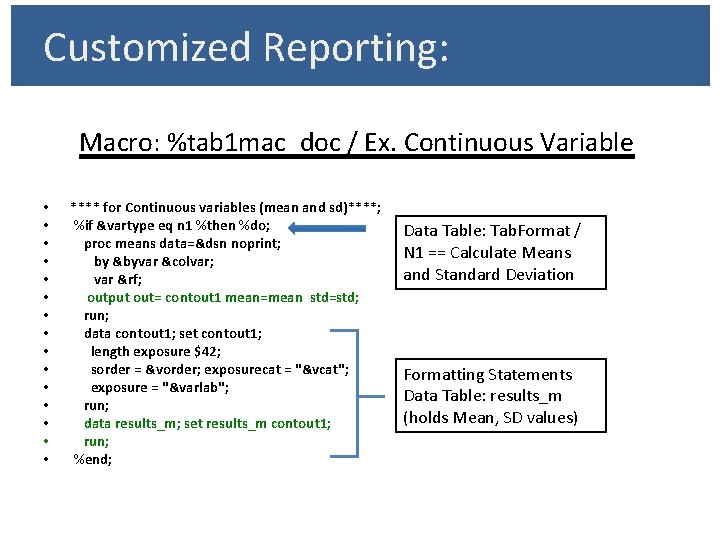
Customized Reporting: Macro: %tab 1 mac_doc / Ex. Continuous Variable • • • • **** for Continuous variables (mean and sd)****; %if &vartype eq n 1 %then %do; proc means data=&dsn noprint; by &byvar &colvar; var &rf; output out= contout 1 mean=mean std=std; run; data contout 1; set contout 1; length exposure $42; sorder = &vorder; exposurecat = "&vcat"; exposure = "&varlab"; run; data results_m; set results_m contout 1; run; %end; Data Table: Tab. Format / N 1 == Calculate Means and Standard Deviation Formatting Statements Data Table: results_m (holds Mean, SD values)

Customized Reporting: Macro: %tab 1 mac_doc / Proc Report Statements • • • Generate a dataset tabres – combination of all results (continuous, categorical). Formatting of table columns for final report %if (&byvar ne ) and (&vcat ne ) %then %do; ods listing; ods document name=&libout. . &fileout (write); proc report data=tabres nowd headline ps=130; column exposurecat exposure &byvar, &colvar, freqpercent ; define exposurecat/ group order=data width=55; define exposure /group order=data width=45; define &byvar /across order=internal format=byf. ; define &colvar /across order=internal format=colf. ; define freqpercent /group width=20; run; ods document close; run; quit; %end;

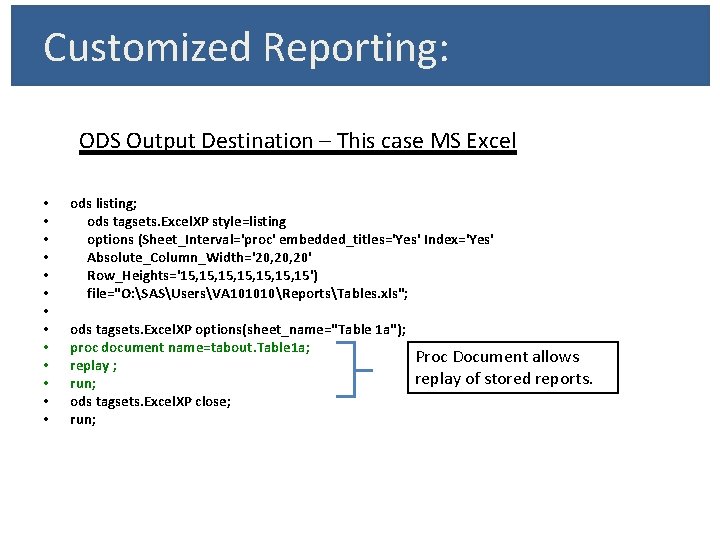
Customized Reporting: ODS Output Destination – This case MS Excel • • • • ods listing; ods tagsets. Excel. XP style=listing options (Sheet_Interval='proc' embedded_titles='Yes' Index='Yes' Absolute_Column_Width='20, 20' Row_Heights='15, 15, 15, 15') file="O: SASUsersVA 101010ReportsTables. xls"; ods tagsets. Excel. XP options(sheet_name="Table 1 a"); proc document name=tabout. Table 1 a; Proc Document allows replay ; replay of stored reports. run; ods tagsets. Excel. XP close; run;

DCF Report

Concluding Remarks: § Centralized monitoring activity can improve efficiency of multi-center trials. • Part of a Risk-Based approach to monitoring. § e. DC applications contains operational tables that can assist in these activities. • Beyond defined reports in e. DC application. § SAS / ODS Reporting Features can be used to generate professional reports. • Modular reporting macro can assist in providing center metrics.

Concluding Remarks: § Full Macro Information • Available at (http: //people. bu. edu/gagnon) § Acknowledgements • SAS® Acknowledgements Ø SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration. • Co-authors: Ø Rebecca Fink / David Gagnon • Staff at VA CSP Boston Coordinating Center / MAVERIC Ø Erika Holmberg / Allan Lewis Audience Questions
 Clinical trial financial management
Clinical trial financial management Cynchia
Cynchia Dicompi
Dicompi Morpheus bms
Morpheus bms Clinical trial budget example
Clinical trial budget example Novel clinical drug trial design
Novel clinical drug trial design Clinical trial api
Clinical trial api Phase 4 trial
Phase 4 trial Nida clinical trial network
Nida clinical trial network Companion diagnostic regulation
Companion diagnostic regulation Clinical trial exports
Clinical trial exports Clinical trial worksheet
Clinical trial worksheet Master clinical trial agreement
Master clinical trial agreement Clinical trial matching service
Clinical trial matching service Accelerated clinical trial agreement template
Accelerated clinical trial agreement template Trofinetide rett
Trofinetide rett Accelerated clinical trial agreement acta
Accelerated clinical trial agreement acta Mosaico clinical trial
Mosaico clinical trial Clinical trials prs
Clinical trials prs Clinical trial centers alliance
Clinical trial centers alliance Ivr iwr studies
Ivr iwr studies Sas clinical standards toolkit
Sas clinical standards toolkit Swimmer plot sas
Swimmer plot sas Can you use the sss postulate or the sas postulate to prove
Can you use the sss postulate or the sas postulate to prove Fspos
Fspos Typiska novell drag
Typiska novell drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Returpilarna
Returpilarna



















































