Unit 3 Topic 5 Chemical Reactions Chemical reactions





- Slides: 20

Unit 3 Topic 5

Chemical Reactions Chemical reactions can be described by writing balanced chemical equations A balanced chemical equation shows the formulas of both the reactants (what you start with) and the products (what was made), the mole ratio of how they interact and their physical states

Products and Reactants The reactants are placed on the left-hand side of the reaction arrow And the products are placed on the righthand side of the reaction arrow Reactants Products

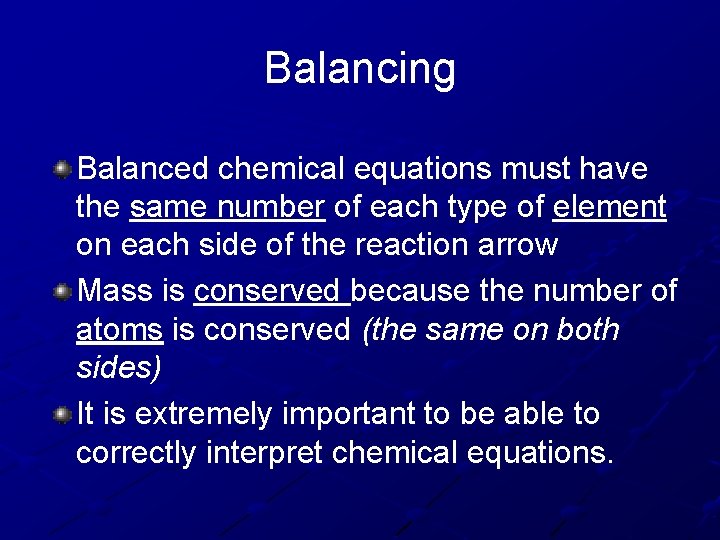
Balancing Balanced chemical equations must have the same number of each type of element on each side of the reaction arrow Mass is conserved because the number of atoms is conserved (the same on both sides) It is extremely important to be able to correctly interpret chemical equations.

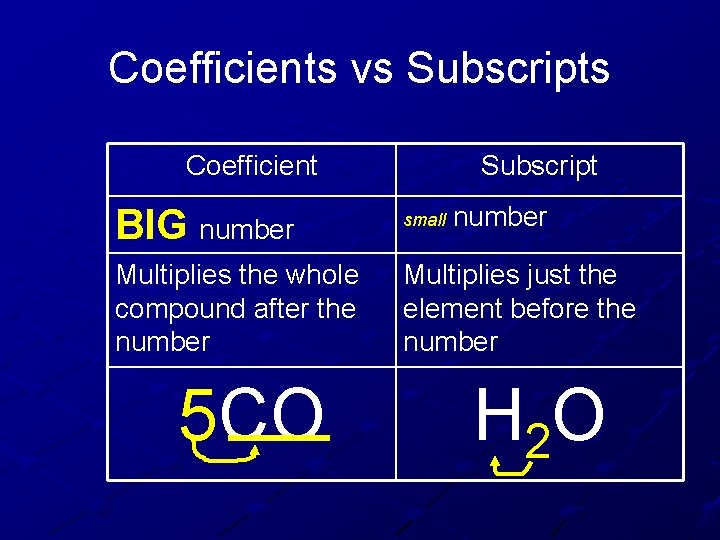
Coefficients vs Subscripts Coefficient Subscript number BIG number small Multiplies the whole compound after the number Multiplies just the element before the number 5 CO H 2 O

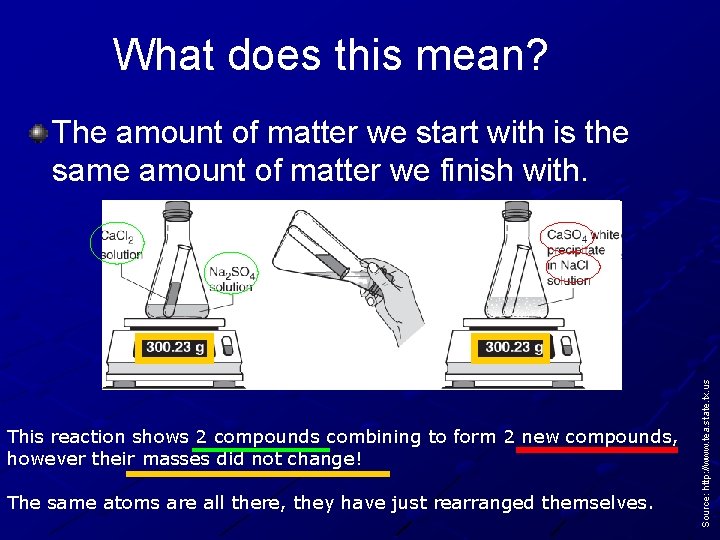
What does this mean? This reaction shows 2 compounds combining to form 2 new compounds, however their masses did not change! The same atoms are all there, they have just rearranged themselves. Source: http: //www. tea. state. tx. us The amount of matter we start with is the same amount of matter we finish with.

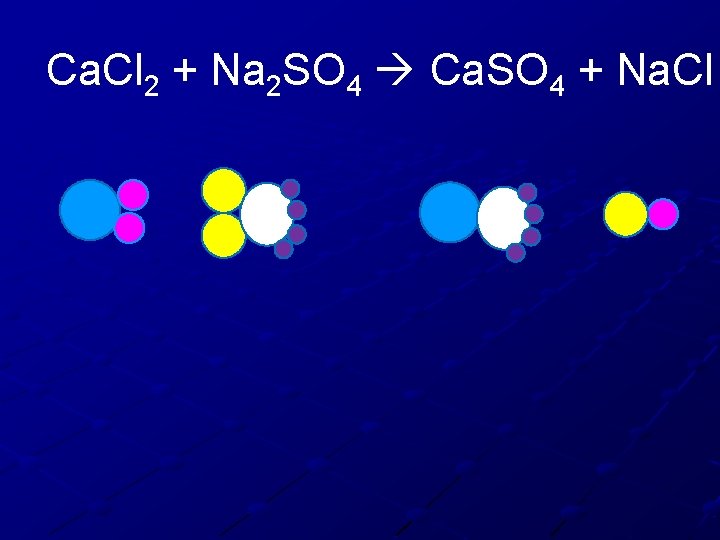
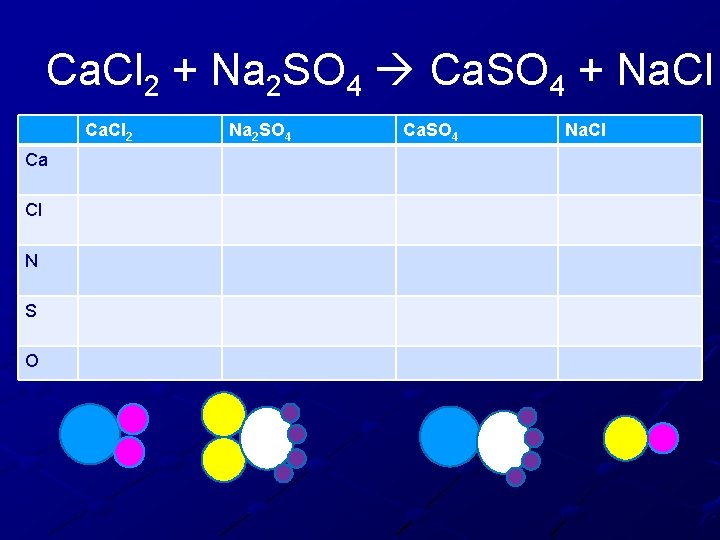
Ca. Cl 2 + Na 2 SO 4 Ca. SO 4 + Na. Cl

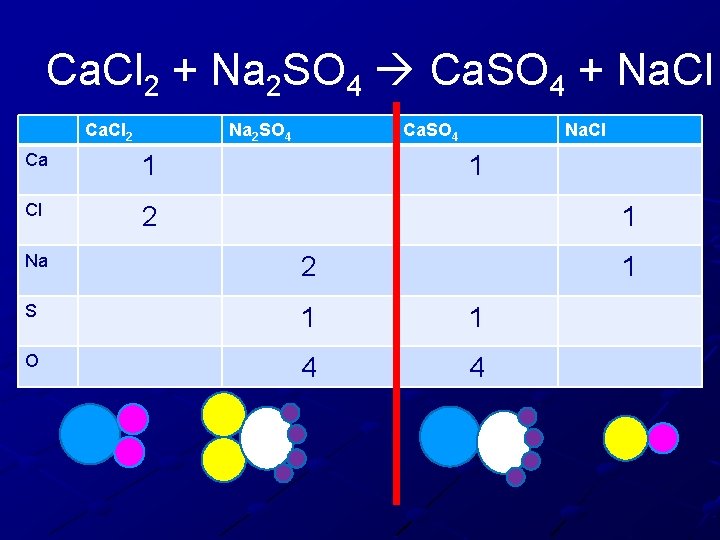
Ca. Cl 2 + Na 2 SO 4 Ca. SO 4 + Na. Cl Ca. Cl 2 Ca Cl N S O Na 2 SO 4 Ca. SO 4 Na. Cl

Ca. Cl 2 + Na 2 SO 4 Ca. SO 4 + Na. Cl Ca. Cl 2 Na 2 SO 4 Ca 1 Cl 2 Ca. SO 4 Na. Cl 1 1 Na 2 S 1 1 O 4 4 1

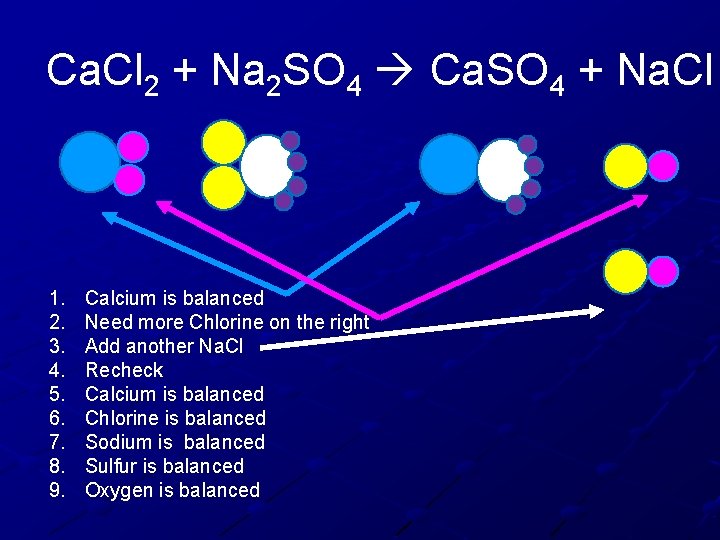
Ca. Cl 2 + Na 2 SO 4 Ca. SO 4 + Na. Cl 1. 2. 3. 4. 5. 6. 7. 8. 9. Calcium is balanced Need more Chlorine on the right Add another Na. Cl Recheck Calcium is balanced Chlorine is balanced Sodium is balanced Sulfur is balanced Oxygen is balanced

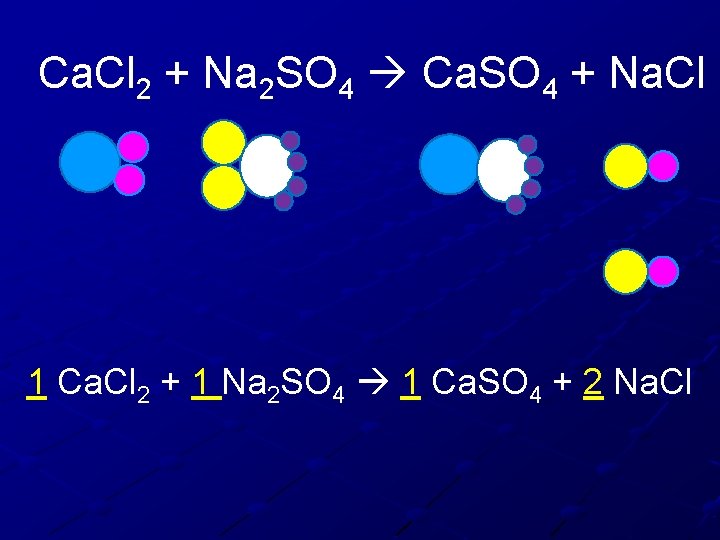
Ca. Cl 2 + Na 2 SO 4 Ca. SO 4 + Na. Cl 1 Ca. Cl 2 + 1 Na 2 SO 4 1 Ca. SO 4 + 2 Na. Cl

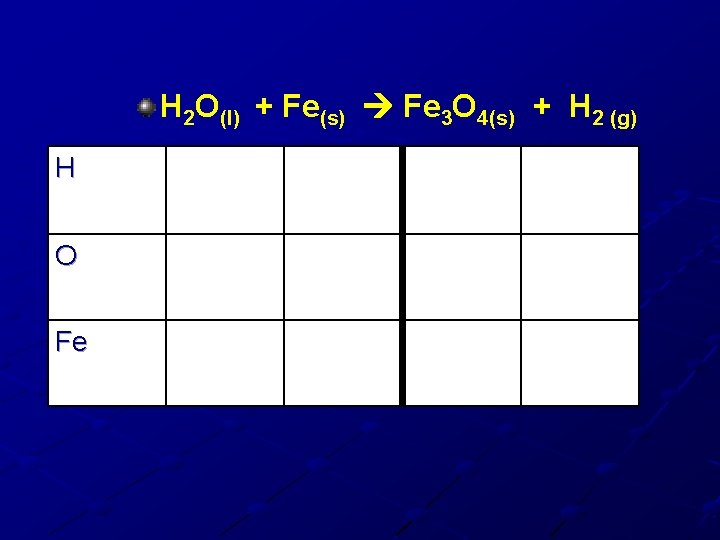
H 2 O(l) + Fe(s) Fe 3 O 4(s) + H 2 (g) H O Fe

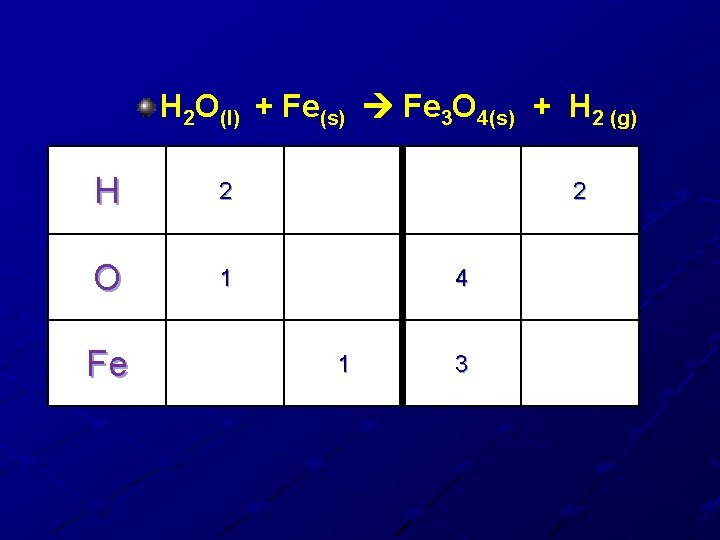
H 2 O(l) + Fe(s) Fe 3 O 4(s) + H 2 (g) H 2 O 1 Fe 2 4 1 3

Is this equation balanced? NO! In order to balance the reaction we need to place coefficients in front of each substance in order to make the number of each element the same

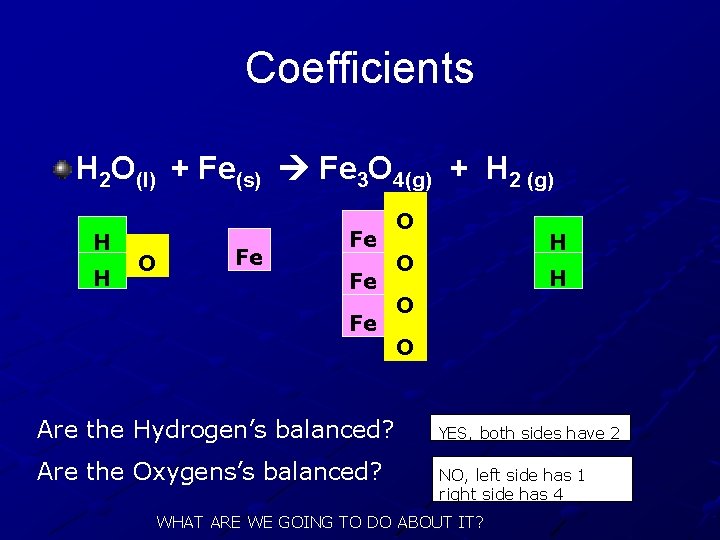
Coefficients H 2 O(l) + Fe(s) Fe 3 O 4(g) + H 2 (g) H H O Fe Fe Are the Hydrogen’s balanced? Are the Oxygens’s balanced? O H O O YES, both sides have 2 NO, left side has 1 right side has 4 WHAT ARE WE GOING TO DO ABOUT IT?

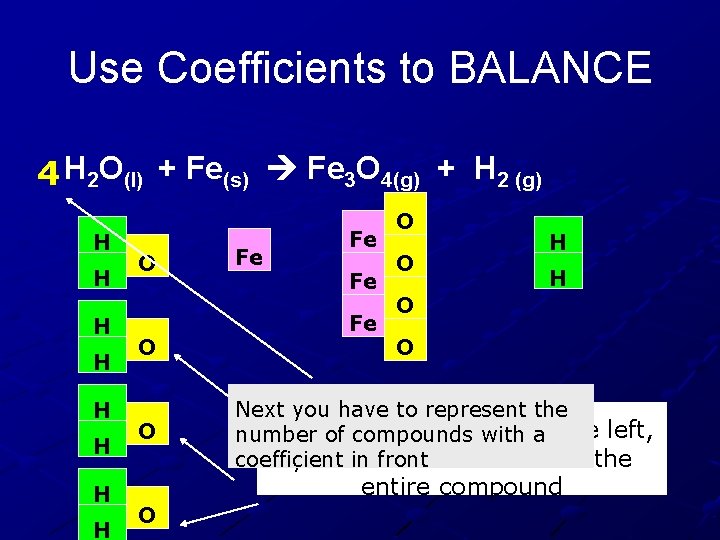
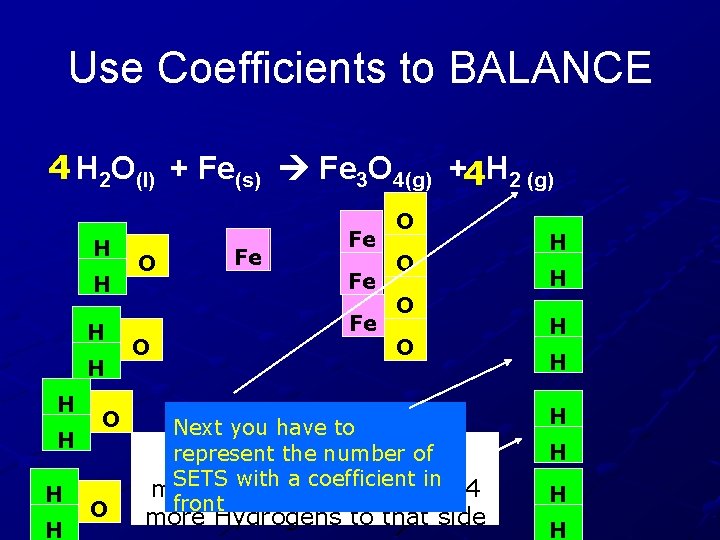
Use Coefficients to BALANCE 4 H 2 O(l) + Fe(s) Fe 3 O 4(g) + H 2 (g) H H H H O O O Fe Fe O O H H O O Next you have to represent the To add more Oxygen number of compounds with to a the left, you have to add more of the coefficient in front entire compound O

Use Coefficients to BALANCE 4 H 2 O(l) + Fe(s) Fe 3 O 4(g) +4 H 2 (g) H H H H O O Fe Fe O O Next you have to represent the number need of Now the Hydrogens SETS with a coefficient in more on the right, so add 4 front more Hydrogens to that side H H H H

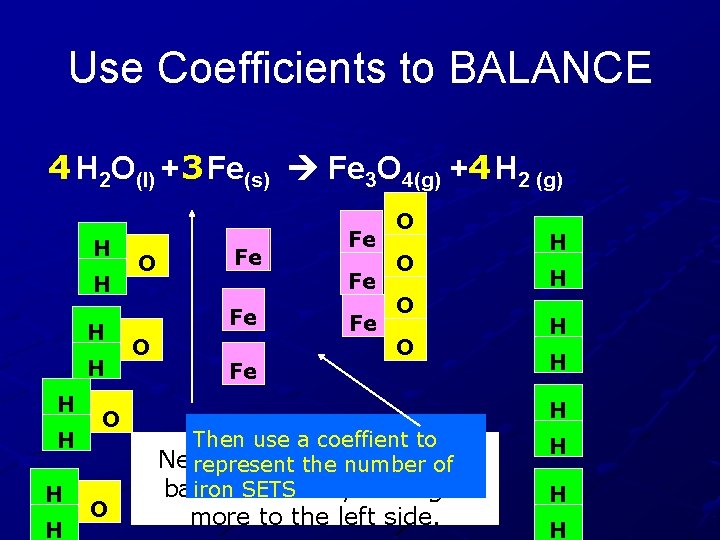
Use Coefficients to BALANCE 4 H 2 O(l) + 3 Fe(s) Fe 3 O 4(g) +4 H 2 (g) H H H H O O O Fe Fe O O H H H Then use a coeffient to Next, the irons need toofbe represent the number balanced out by adding 2 iron SETS more to the left side. H H H

When you finish with the Legos Complete Page 11 and show Mrs. Liller for credit. Next complete pages 12 and 13. What you don’t finish do for homework. Balancing Quiz Friday 11/14 I need to see page 7 and 8 from: Derrick Hevert James William

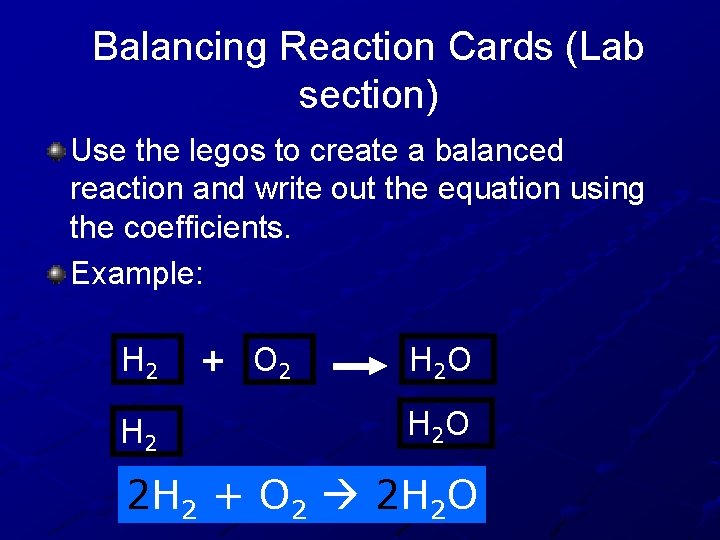
Balancing Reaction Cards (Lab section) Use the legos to create a balanced reaction and write out the equation using the coefficients. Example: H 2 + O 2 H 2 O 2 H 2 + O 2 2 H 2 O
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Unit 5 chemical reactions
Unit 5 chemical reactions Unit 11 chemical reactions
Unit 11 chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Third person example
Third person example Narrowed down topic
Narrowed down topic Example of redox reaction
Example of redox reaction Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test Stoichiometry island diagram
Stoichiometry island diagram Types of chemical reactions redox
Types of chemical reactions redox Types of reactions
Types of reactions Types of reactions chemistry
Types of reactions chemistry 5 types of chemical reactions
5 types of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions







































