Transfusion Medicine Vascular Access for Red Cell Exchange















- Slides: 39

Transfusion Medicine: Vascular Access for Red Cell Exchange Laura Craig-Owens, MD

Sickle Cell Disease and Red Cell Exchange • Sickle cell disease is a major health problem with significant morbidity and mortality. • In many patient’s supportive management is sufficient, however some patient’s need more aggressive therapy such as red cell exchange. • The majority of transfusions for sickle cell disease are simple red cell transfusions. These transfusions add oxygen carrying capacity. • Unless there is bleeding or rapid cell destruction, the hemoglobin concentration and viscosity of the blood is increased. Swerdlow P S Hematology 2006; 2006: 48 -53

Sickle Cell Disease and Red Cell Exchange • The viscosity of blood is a key concept in the management of transfusions in sickle cell disease. • The blood viscosity increases with increasing hemoglobin levels and limits blood flow and oxygen transport. • Deoxygenated sickle cells have nearly a 10 fold greater viscosity than oxygenated sickle cells at the same hemoglobin level. Swerdlow P S Hematology 2006; 2006: 48 -53

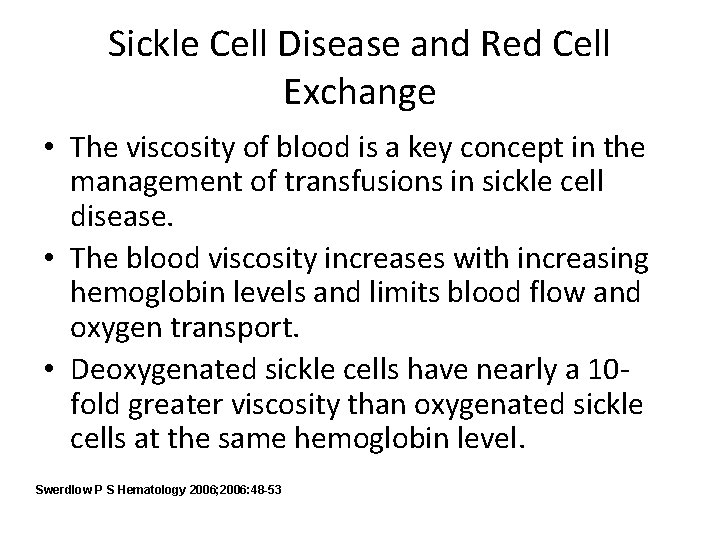
Sickle Cell Disease and Red Cell Exchange • Transfusing normal red blood cells to individuals with sickle cell disease increase the viscosity. • The following diagram shows oxygen transport vs. hemoglobin levels. Swerdlow P S Hematology 2006; 2006: 48 -53

Sickle Cell Disease and Red Cell Exchange • Red cell exchanges can administer blood without a corresponding increase in viscosity. • Red cell exchanges are used to not only increase oxygen carrying capacity but to also decrease complications of sickle cell disease. • This procedure is indicated in primary and secondary vaso-occlusive stroke prevention, acute chest syndrome, multiorgan failure syndrome, and chronic maintenance of low Hgb S levels. Swerdlow P S Hematology 2006; 2006: 48 -53

Sickle Cell Disease and Red Cell Exchange • Red cell exchange has been used in patients who are to undergo major surgeries to decrease incidence of postsurgical complications and individuals without sickle cell disease who have parasitemia of the blood due to malaria. • Other indications for red cell exchange with less supporting evidence are elements of the triad of pulmonary hypertension, priapism, and leg ulcers associated with red cell hemolysis, chronic hypoxia not controllable with oxygen therapy, repeated episodes of acute chest syndrome or multiorgan failure syndrome, or multiple severe pain episodes interfering with major life goals. Swerdlow P S Hematology 2006; 2006: 48 -53

Sickle Cell Disease and Red Cell Exchange • In red cell exchange, sickle cells are replaced with normal cells. This can prevent further vaso-occlusion in individuals, but cannot reverse pre-existing vasoocclusion. • It can also rapidly decrease the rate of hemolysis of red cells which can decrease liver processing of bilirubin, damage to renal tubular cells and scavenging of nitric oxide by free hemoglobin released from sickle cells. • The goal of exchange is to have a Hgb S less than 30% and a hemoglobin close to but not greater than 10. Swerdlow P S Hematology 2006; 2006: 48 -53

Sickle Cell Disease and Red Cell Exchange • In order to perform a red cell exchange, a patient must have appropriate vascular access. • Peripheral veins, indwelling lines, and ports may be used for the procedure. • Most venous access devices do not have the ability to support the flow rates needed for apheresis machines. Swerdlow P S Hematology 2006; 2006: 48 -53

Central Venous Access and Sickle Cell Disease • Patients with sickle cell disease are prone to develop thrombosis and infections due to their inflammatory and immune deficiency. • These patients tend to have issues with vascular access and require central venous access catheterization. • An ideal apheresis catheter should have two lumens, should not allow recirculation, and should allow blood flow of 150 ml/min. • A diameter of 10 -13. 5 F allows for adequate flow for an apheresis patient. Yeral, Mahmut, 2014; 37: 97 -101

Central Venous Access and Sickle Cell Disease • Catheters should be removed as early as possible due to the high risk of complications in SCD. • Extended implant time increases the risk of thrombosis and infection. • Therefore patients who are going to be receiving chronic therapy may have implanted ports placed. Yeral, Mahmut, 2014; 37: 97 -101

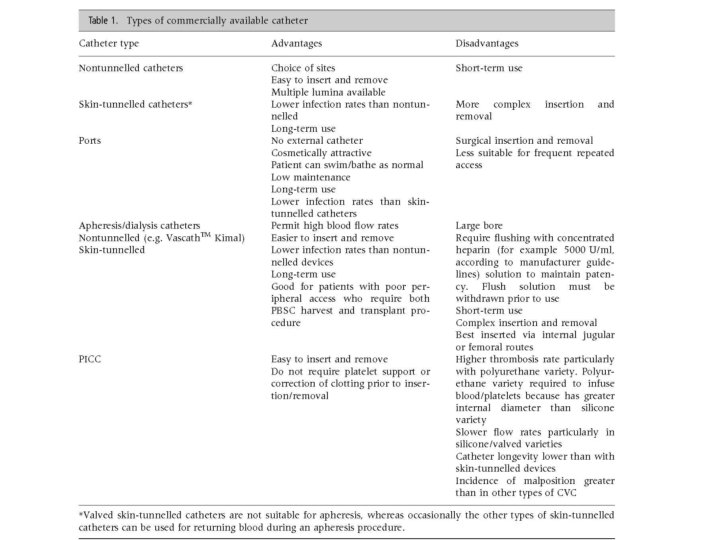
Central Venous Access Devices • Central venous access devices are categorized into: – Nontunneling catheters – Tunneling catheters – Implanted ports – Apheresis/dialysis catheters – PICC lines L. Bishop et al, 2007; 29: 261 -278

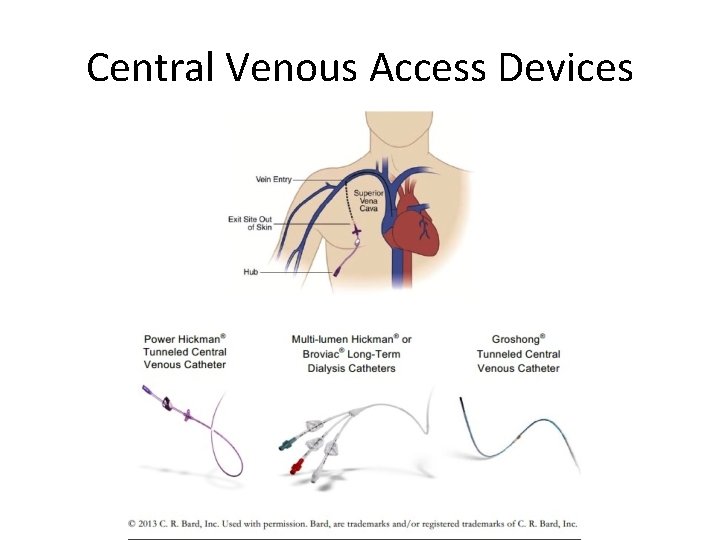
Central Venous Access Devices • Nontunneled central venous catheters are fixed in place at the site of the insertion, with the catheter and attachments protruding directly. • Example: Quinton catheter • This type of catheter is recommended for those who require short term (<10 days) central venous catheterization or who are at high risk for infection. L. Bishop et al, 2007; 29: 261 -278

Central Venous Access Devices Quinton Catheter

Central Venous Access Devices • Tunneled catheters are used in patients in whom long term (>30 days) central venous access is necessary. This type of catheter is passed under the skin from an insertion site to a separate exit site where the catheter and attachments emerge. • Examples: Hickman and Groshong catheters • They exist with and without cuffs. The cuff induces an inflammatory response within the subcutaneous tunnel leading to fibrosis. The catheter becomes fixed within 3 -4 weeks following insertion. • These catheters have been associated with lower infection risks than nontunneled catheters. • These catheters are not ideal for apheresis due to their soft walls which collapse with the vacuum pull of the pheresis machines L. Bishop et al, 2007; 29: 261 -278

Central Venous Access Devices Groshong

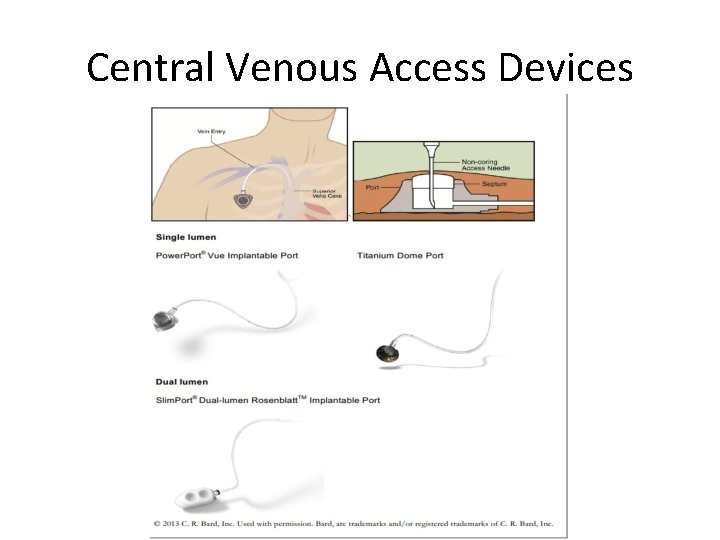
Central Venous Access Devices • Implanted ports are similar to tunneled catheters except they are left entirely under the skin. The distal end of the catheter is formed by a small metal reservoir which is covered by a membrane. This membrane must be accessed by a needle. • They tend to be used more frequently in pediatric patients, patients with solid tumors, individuals with sickle cell anemia and thalassemia, and oncology patients. • Ports have the lowest reported rates of catheter related blood stream infections compared with nontunneled and tunneled central venous catheters (CVC). • They also allow less restricted bathing and swimming. They appeal to patients who are concerned about the appearance of external parts of nonimplanted catheters. • They are more expensive to purchase, insert and remove, and leave larger scars. L. Bishop et al, 2007; 29: 261 -278

Central Venous Access Devices

Central Venous Access Devices • Vortex ports: – This port is available in plastic or titanium. – It is set at a tangent rather than perpendicular which helps to create a flushing action within the port to hyper cleanse the chamber to decrease the amount of sludge, build-up, and reduced rate of occlusion. – Occlusion in vortex ports was found to be 7% compared to ports without tangential outlets (27%) according to one randomized control trial. Goossens, GA, et al, 2008; 16: 1367 -1374

Central Venous Access Devices http: //msa. com. au/product/vascular-access/vortex-port/

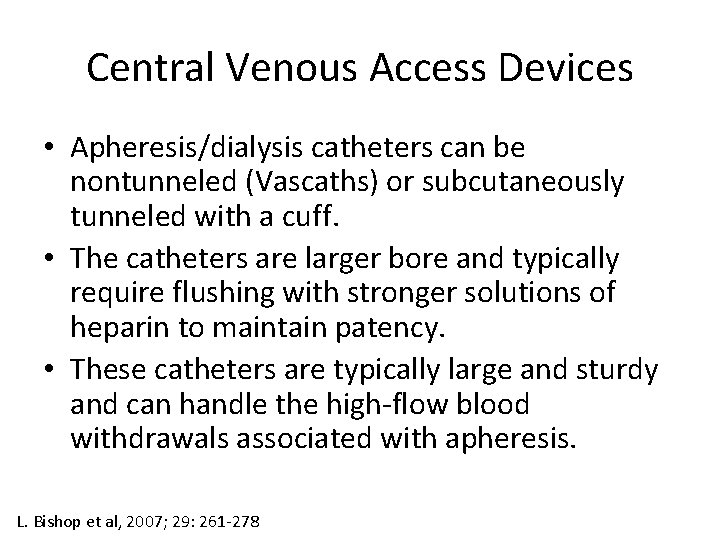
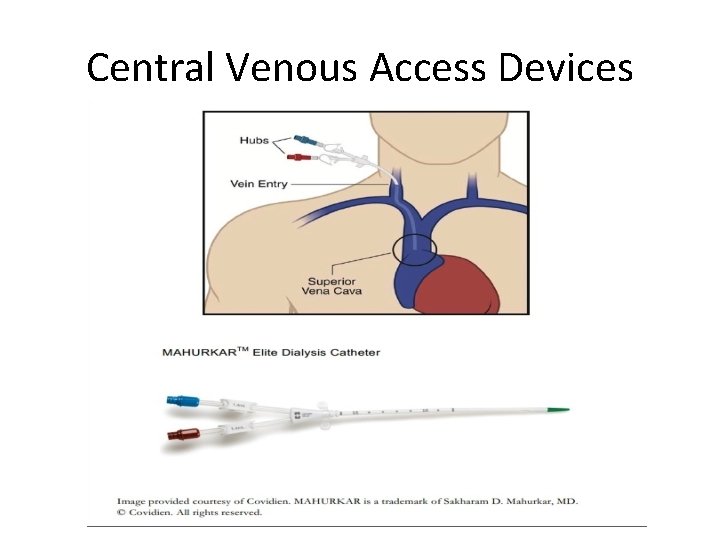
Central Venous Access Devices • Apheresis/dialysis catheters can be nontunneled (Vascaths) or subcutaneously tunneled with a cuff. • The catheters are larger bore and typically require flushing with stronger solutions of heparin to maintain patency. • These catheters are typically large and sturdy and can handle the high-flow blood withdrawals associated with apheresis. L. Bishop et al, 2007; 29: 261 -278

Central Venous Access Devices

Central Venous Access Devices • PICC lines are inserted in the veins of the arm. • These catheters are suited for ambulatory or outpatient therapy. L. Bishop et al, 2007; 29: 261 -278


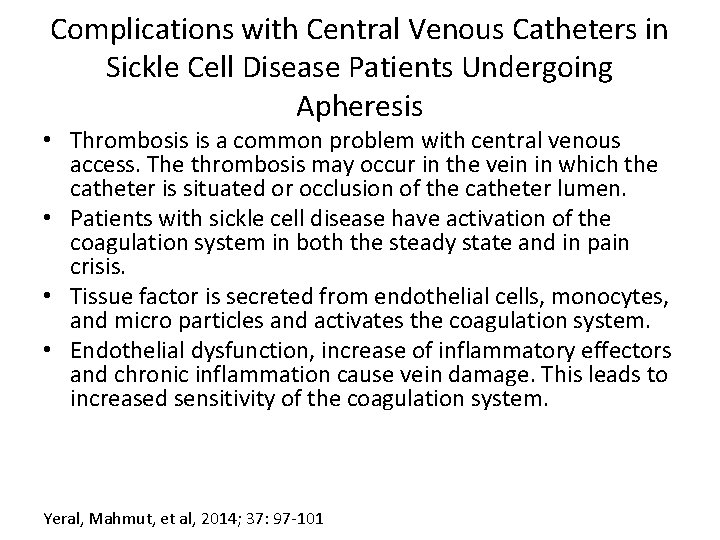
Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis • Thrombosis is a common problem with central venous access. The thrombosis may occur in the vein in which the catheter is situated or occlusion of the catheter lumen. • Patients with sickle cell disease have activation of the coagulation system in both the steady state and in pain crisis. • Tissue factor is secreted from endothelial cells, monocytes, and micro particles and activates the coagulation system. • Endothelial dysfunction, increase of inflammatory effectors and chronic inflammation cause vein damage. This leads to increased sensitivity of the coagulation system. Yeral, Mahmut, et al, 2014; 37: 97 -101

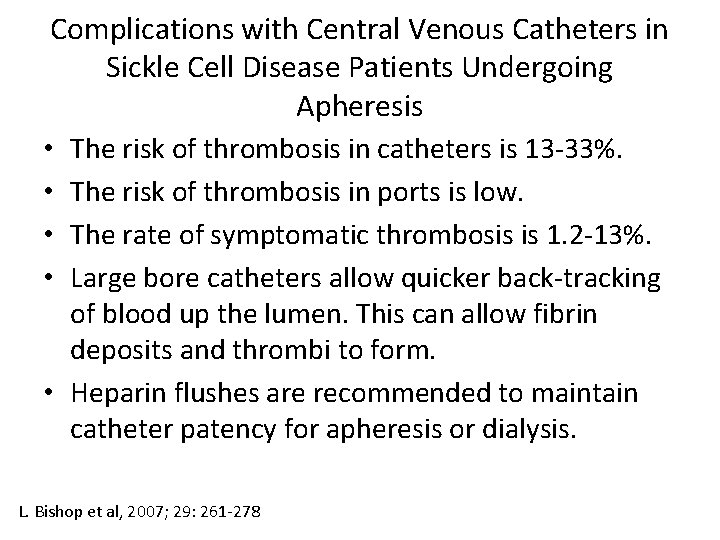
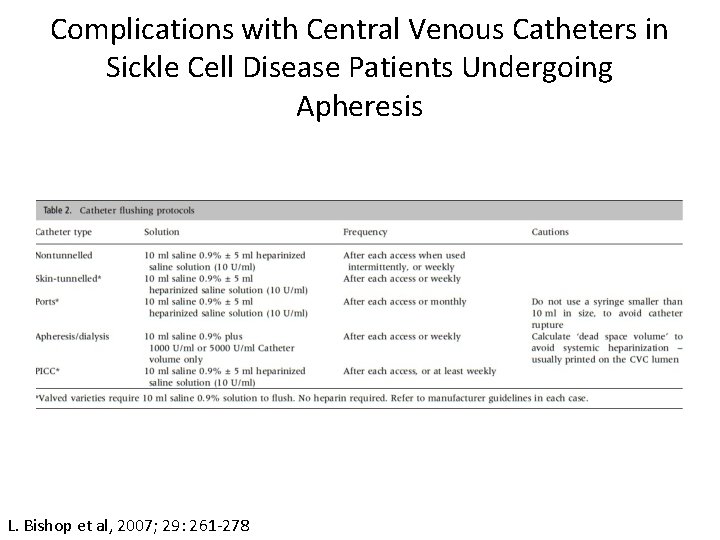
Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis • The risk of thrombosis in catheters is 13 -33%. • The risk of thrombosis in ports is low. • The rate of symptomatic thrombosis is 1. 2 -13%. • Large bore catheters allow quicker back-tracking of blood up the lumen. This can allow fibrin deposits and thrombi to form. • Heparin flushes are recommended to maintain catheter patency for apheresis or dialysis. L. Bishop et al, 2007; 29: 261 -278

Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis L. Bishop et al, 2007; 29: 261 -278

Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis • Anticoagulation with warfarin can also be used in patients with permanent fixtures unless there is contraindication for anticoagulation. • Another option for clotted catheter lumens is to use urokinase or t-PA. Only intraluminal volumes should be instilled into the catheter. • This solution should be injected gently into the catheter with a push-pull action to maximize mixing with the lumen. • The lumen should then be clamped and left for 2 -3 hours. The catheter should then be unclamped and the solution containing the disaggregated blood aspirated. L. Bishop et al, 2007; 29: 261 -278

Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis • Infection is also a common complication associated with central venous access and individuals with sickle cell disease (SCD). • The incidence of infections encountered by those with SCD is multifactorial. • Patients with SCD are predisposed to infection from encapsulated organisms (S. pneumonia, Salmonella, and H. influenza) because of autosplenectomy and defective opsonization. Alkindi, Salam, 2012, 5: 57 -62

Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis • Also increased risk of infection may be due to iron overload and altered phagocyte function due to repeated transfusions. • Inappropriate care of catheters and ports as well as their misuse with injections of recreational drugs can lead to infection. Alkindi, Salam, 2012, 5: 57 -62

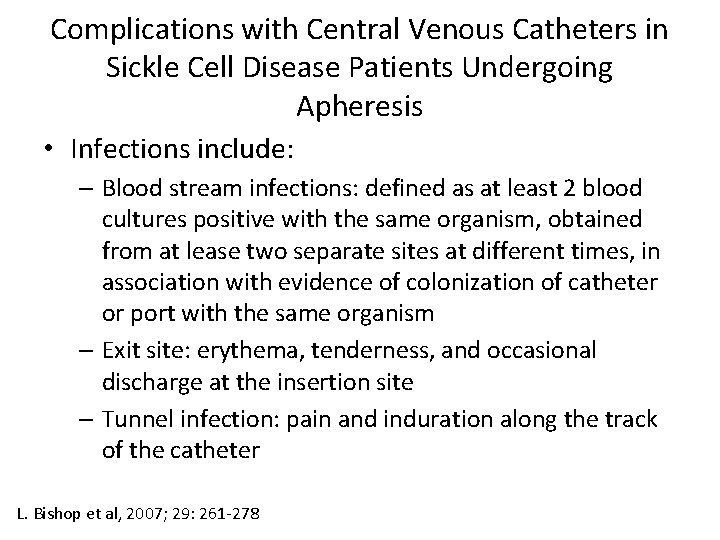
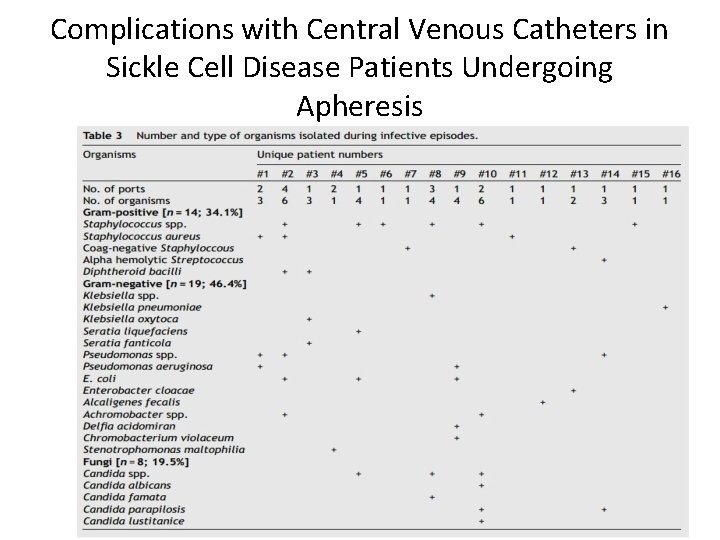
Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis • Infections include: – Blood stream infections: defined as at least 2 blood cultures positive with the same organism, obtained from at lease two separate sites at different times, in association with evidence of colonization of catheter or port with the same organism – Exit site: erythema, tenderness, and occasional discharge at the insertion site – Tunnel infection: pain and induration along the track of the catheter L. Bishop et al, 2007; 29: 261 -278

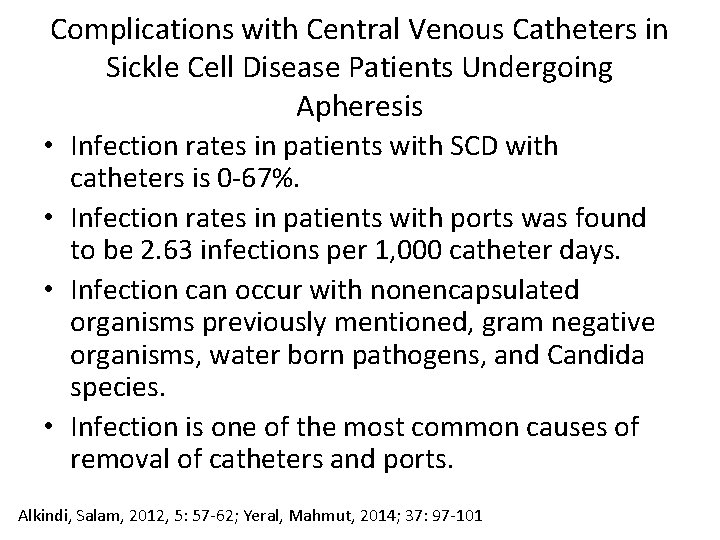
Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis • Infection rates in patients with SCD with catheters is 0 -67%. • Infection rates in patients with ports was found to be 2. 63 infections per 1, 000 catheter days. • Infection can occur with nonencapsulated organisms previously mentioned, gram negative organisms, water born pathogens, and Candida species. • Infection is one of the most common causes of removal of catheters and ports. Alkindi, Salam, 2012, 5: 57 -62; Yeral, Mahmut, 2014; 37: 97 -101

Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis

Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis • Catheter malfunction: – Partial or complete blockage of lumen followed by forcible introduction of fluid may cause catheter rupture – Catheter occlusion can include blockage resulting from kinking of the catheter, pinch off syndrome, occlusion of the catheter tip on the vessel wall, or migration of the tip into a smaller vessel. L. Bishop et al, 2007; 29: 261 -278

Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis • When catheter malfunction occurs the catheter may cease to function. • A chest x-ray may help to diagnose this problem. • When these problems occur the catheter may need to be removed because internal repair of catheters is no recommended due to risk of infection and introduction of an air embolus. L. Bishop et al, 2007; 29: 261 -278

Complications with Central Venous Catheters in Sickle Cell Disease Patients Undergoing Apheresis • Port malfunction: – Mechanical occlusion: due to surgical technique (pinch off, catheter tip malposition); poor device management leading to deposits of minerals lipids, and drug precipitates in the port and catheter; patient related factors – Thrombotic occlusion: intra-luminal thrombus which causes withdrawal occlusion which is the inability to withdraw blood from the system while infusion is still possible. This occurs in 5. 8 -26% of ports. • It is reported that nurse spend an extra 27. 1 -29 minutes troubleshooting problems accessing the port when there is occlusion Goossens, GA, et al, 2008; 16: 1367 -1374

Ports at our Institution-Discussion with Interventional Radiology (IR) • IR was previously placing Vortex ports. These have been found to not be as durable due to their silicone catheters. The catheters are 11 F in size (1 F=0. 3 mm). They are using this port less frequently because of its durability and also because it is not power injectable. • Power port duo is what IR is now placing more frequently. This port is a 9 F polyurethane catheter. Even though the size of the catheter is smaller the intraluminal size is larger. – Important to note: Dual sides of the port: lateral side for aspiration and medial side for return

Ports at our Institution-Discussion with Interventional Radiology (IR) • Previously used Dialoc ports for dialysis. These are no longer used because they were more prone to clotting due to their high frequency use. However, could these be more useful in sickle cell patients receiving red cell exchange once a month? • IR is wanting to do a study looking at current red cell exchange patients and the ports that are being used. • IR is willing to be called when there are problems with access and want to help with a protocol.

Discussions with Apheresis/Dialysis at Our Own Institution • Who is responsible for care of lines and ports? • Can a resident or attending be present during access for the procedure? • Ports tend to be difficult for nurses to access because of being clotted off • Is there a plan for patient’s whose ports are not working but need a red cell exchange?

References • • Alkindi, Salam, et al. Complications of PORT-A-CATH in patients with sickle cell disease. Journal of Infection and Public Health, 2012; 5: 57 -62. Bishop, L. Guidelines on the insertion and management of central venous access devices in adults. Int Jnl Lab Hem, 2007; 29: 261 -278. Goossens, G. A. , et al. Functional evaluation of conventional Celsite venous ports versus Vortex ports with tangential outlet: a prospective randomized pilot study. Support Care Cancer. 2008; 16: 1367 -1374. Lawson, S. E. , et al. Red cell exchange in sickle cell disease. Clin Lab Hem, 1999; 99102. Raj, Ashok, et al. Cathlink 20: A subcutaneous implanted central venous access device used in children with sickle cell disease on long-term erythrocytapheresis. A report of low complication rates. Pediatr Blood Cancer, 2005; 44: 669 -672. Swerdlow, Paul S. Red cell exchange in sickle cell disease. Hematology, 2006; 48 -53. Yeral, Mahmut, et al. Short-term central venous catheter complications in patients with sickle cell disease who undergo apheresis. J Thrombolysis, 2014; 37: 97 -101.
 Vascular plants vs nonvascular plants
Vascular plants vs nonvascular plants Life cycle of seedless plants
Life cycle of seedless plants Vascular and non vascular difference
Vascular and non vascular difference Chonicu
Chonicu Double volume exchange transfusion
Double volume exchange transfusion Exchange transfusion blood group selection
Exchange transfusion blood group selection Exchange transfusion
Exchange transfusion Determination of exchange rate
Determination of exchange rate Pearl exchange activity
Pearl exchange activity Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Terminal access controller access control system
Terminal access controller access control system Terminal access controller access-control system
Terminal access controller access-control system Black market peso exchange examples
Black market peso exchange examples Hemolytic transfusion reaction
Hemolytic transfusion reaction Transfusion en urgence vitale immédiate
Transfusion en urgence vitale immédiate Blood transfusion indications
Blood transfusion indications Indications for blood transfusion
Indications for blood transfusion Blood bag discarded in
Blood bag discarded in Blood transfusion requirements
Blood transfusion requirements Ffp vs platelets
Ffp vs platelets Twin-twin transfusion syndrome
Twin-twin transfusion syndrome Blood transfusion complications
Blood transfusion complications Blood transfusion complications
Blood transfusion complications Transfusion plaquetas indicaciones
Transfusion plaquetas indicaciones Indications for platelet transfusion
Indications for platelet transfusion Complications of blood transfusion
Complications of blood transfusion Platelets transfusion indication
Platelets transfusion indication Complications of blood transfusion
Complications of blood transfusion Indication of transfusion
Indication of transfusion Blood transfusion requirements
Blood transfusion requirements Fresh frozen plasma transfusion time
Fresh frozen plasma transfusion time French size in mm
French size in mm Massive transfusion complication
Massive transfusion complication Abo system of blood grouping
Abo system of blood grouping Indications for blood transfusion
Indications for blood transfusion Blood grouping and crossmatching
Blood grouping and crossmatching Fresh frozen plasma transfusion time
Fresh frozen plasma transfusion time Effects of blood transfusion
Effects of blood transfusion Cryoprecipetate
Cryoprecipetate Cryopercipitate
Cryopercipitate