Title Simultaneous identification of Mycoplasma Gallisepticum and Mycoplasma







![Reference [1] Bardbury J. M; Kleven, S. H. (2003). Mycoplasma Iowa Infection. in: disease Reference [1] Bardbury J. M; Kleven, S. H. (2003). Mycoplasma Iowa Infection. in: disease](https://slidetodoc.com/presentation_image_h2/8942402624bfb89207674d031871603a/image-32.jpg)
- Slides: 32

Title : Simultaneous identification of Mycoplasma Gallisepticum and Mycoplasma Synoviae by Duplex PCR assay Speaker Name: Golbarg Malekhoseini Affiliation: Islamic Azad University, Qom Branch, Qom, Iran Prof and Head: Dr. Seyed Ali Pourbakhsh University : Mycoplasma Reference Laboratory, Razi Vaccine and Serum Research Institute, Karaj, Iran

Table of Content (summary slide) v Introduction v Objectives v Methods and Material v Result & Discussion v Conclusion v Summary v References 28. 3. 2017 2

Introduction Mycoplasmas are the smallest prokaryote and can pass from 0. 45 micron fillteration. thay donot have cell wall, so they resistant to effective antibiotics on the bacterial wall. ü They have smallest genome (0. 58 -1. 38 Mbp) or (600 -1350 Kb) and independent replication ü They are facultative anaerobic and they need to 10% co 2. ü They provide carbohydrates, lipid, amino acid from their host. They need to colestrol for setting the temperature and fluiding membrance. ü Mycoplasma 16 Sr. RNA analysis showed that they have near relationship with Clastredums and Lactobacilluse. They can cause to disease in plants, animals and insect. 28. 3. 2017 3

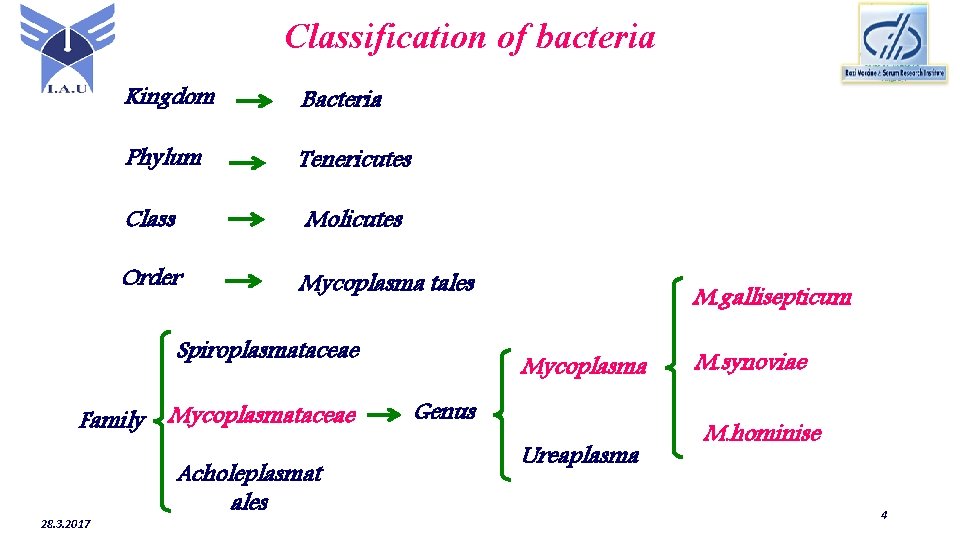
Classification of bacteria Kingdom Bacteria Phylum Tenericutes Class Molicutes Order Mycoplasma tales Spiroplasmataceae Family Mycoplasmataceae 28. 3. 2017 Acholeplasmat ales M. gallisepticum Mycoplasma Genus Ureaplasma M. synoviae M. hominise 4

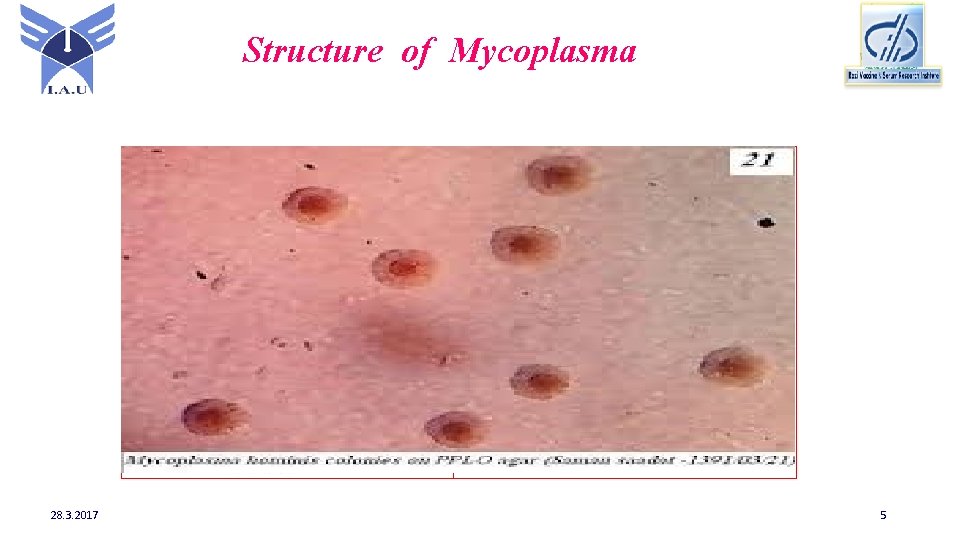
Structure of Mycoplasma 28. 3. 2017 5

Mycoplasma Synoviae & Mycoplasma Gallisepticum Mycoplasma Synoviae(MS) and Mycoplasma Gallisepticum(MG) are two pathogenic species in bird that can cause too economic losses. Infection with MG has different clinical protests may be choronic respiratory disease and broiler carcass quality loss, are the highes incidence MG was known as one of the creator in complex disease and also relation between MG with different respiratory such as(Newcastle virus, Influenza type A), E. coli , Heamophiluse paragalynarum are known. MS is one of the most important pathogens in Turkey and chicken, that causes great economic losses in poultry industry evry year. Although it was introduced as causative agent of infectious synovitis, but nowadays also it 28. 3. 2017 was introduced as a respiratory illness and infection of the air sacs . 6

Objectives Several Mycoplasma can cause Micoplasmosis in poultry. Among them MG and MS are the most important. They can cause economical losses to the poultry industry. Economical losses of Mycoplasma such as breathing problems or infection with virus and bacteria under poor sanitary condition. Till now immonological and molecular methods were used to identify MG and MS. But where as with Duplex PCR we can identify MG and MS at the same time and also we can save time and cost. So we decided to set up this technique for identification MG and MS. 28. 3. 2017 7

Hypothesis There are several discussions such as v Gene that was selected to distinguish MG was 16 Sr. RNA and vlh. A gene is used for identification MS. v MS is more sensetive than MG. So MG is more likely to grow than MS. So it is more likely to identify than MS. v As where as the size MG gene (183 bp) is smaller than MS(350 bp). So MG is more sensetive than MS and also number of MG amplified fragments are more than MS. 28. 3. 2017 8

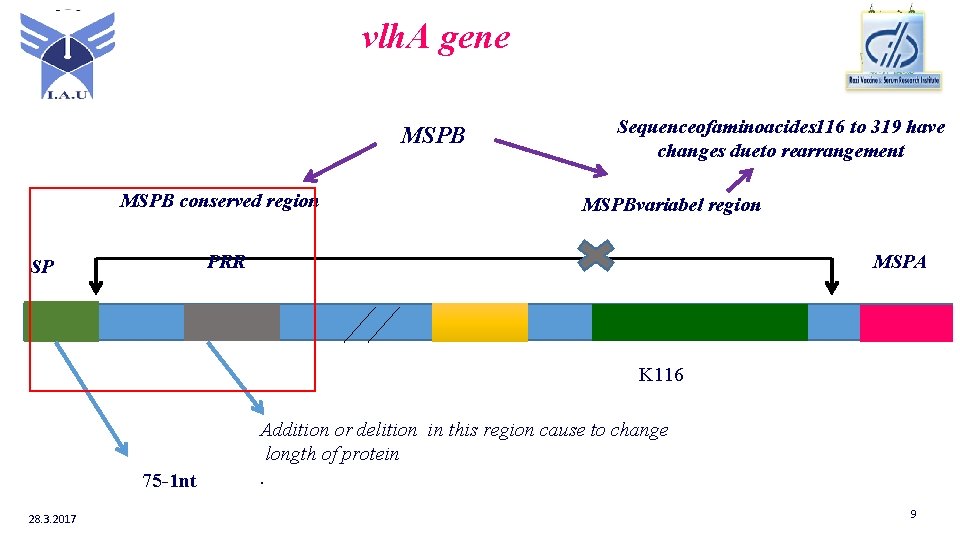
vlh. A gene MSPB conserved region Sequenceofaminoacides 116 to 319 have changes dueto rearrangement MSPBvariabel region PRR SP MSPA K 116 75 -1 nt 28. 3. 2017 Addition or delition in this region cause to change longth of protein. 9

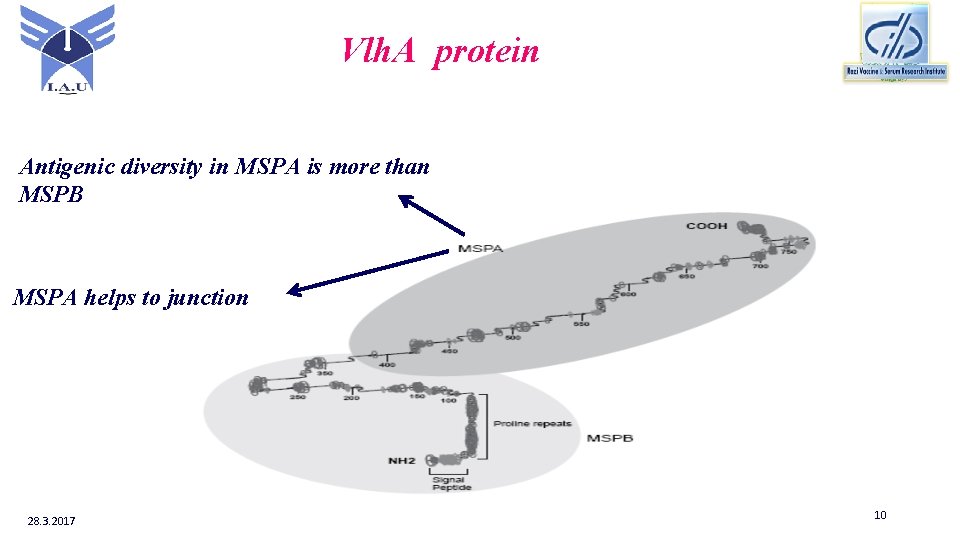
Vlh. A protein Antigenic diversity in MSPA is more than MSPB MSPA helps to junction 28. 3. 2017 10

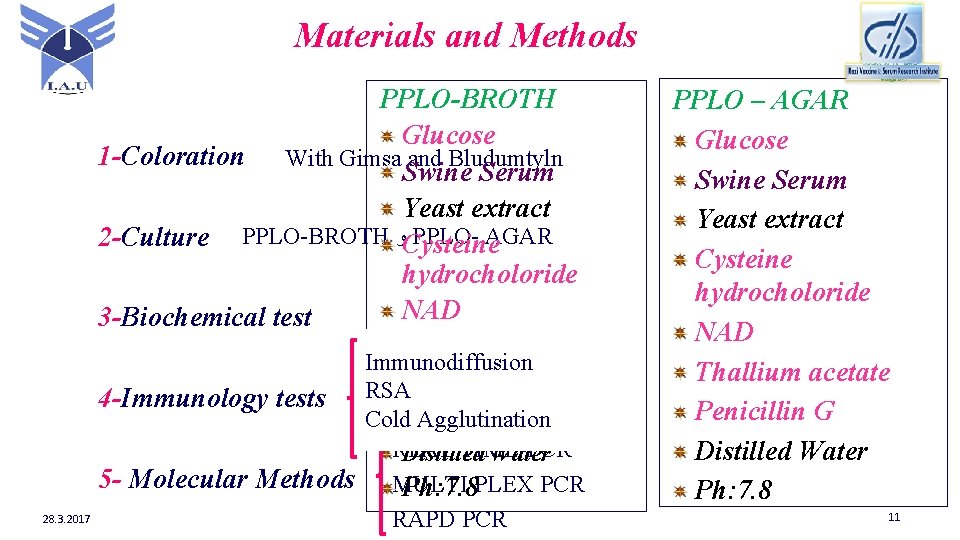
Materials and Methods PPLO-BROTH Glucose 1 -Coloration With Gimsa and Bludumtyln Swine Serum Yeast extract PPLO- AGAR 2 -Culture PPLO-BROTH ﻭ Cysteine hydrocholoride NAD 3 -Biochemical test Phenol red Immunodiffusion Thallium acetate RSA 4 -Immunology tests PCR Cold Agglutination Penicillin G 5 - Molecular Methods 28. 3. 2017 REAL TIME-PCR Distilled Water MULTI Ph: 7. 8 PLEX PCR RAPD PCR PPLO – AGAR Glucose Swine Serum Yeast extract Cysteine hydrocholoride NAD Thallium acetate Penicillin G Distilled Water Ph: 7. 8 11

Molecular Methods 28. 3. 2017 12

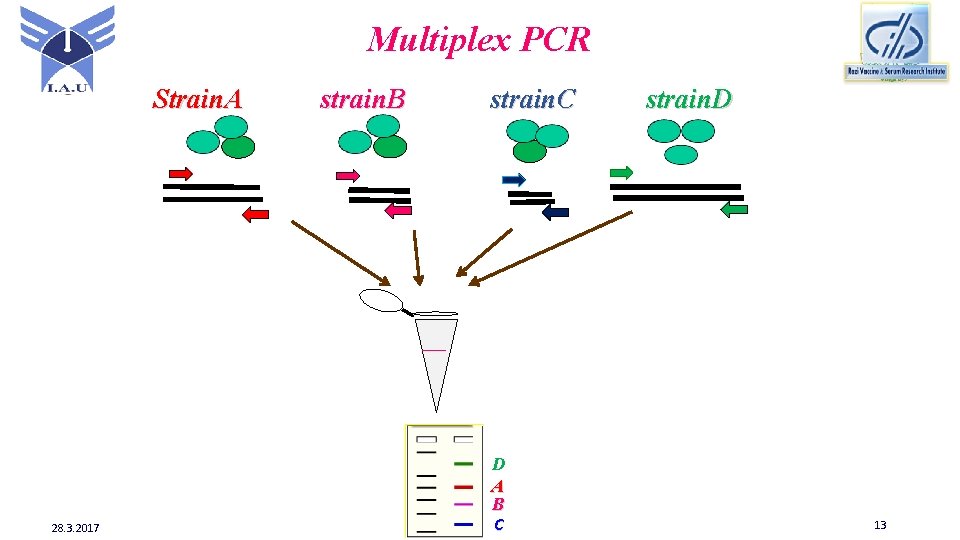
Multiplex PCR Strain. A 28. 3. 2017 strain. B strain. C D A B C strain. D 13

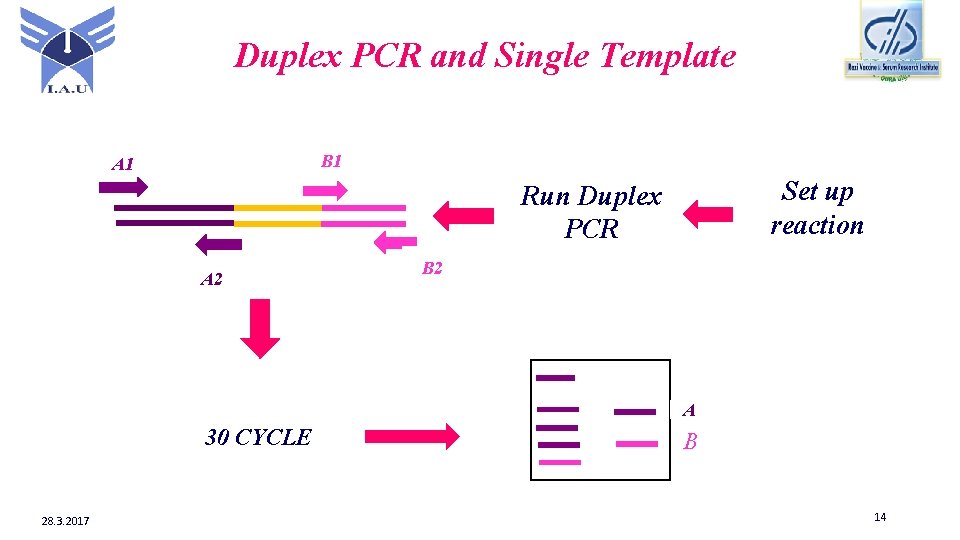
Duplex PCR and Single Template B 1 A 1 Set up reaction Run Duplex PCR A 2 B 2 A 30 CYCLE 28. 3. 2017 B 14

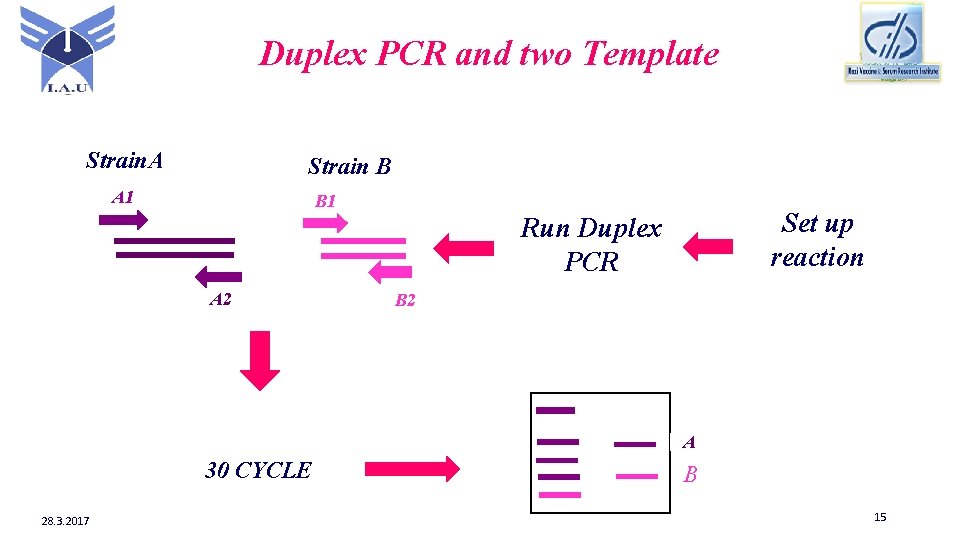
Duplex PCR and two Template Strain. A Strain B A 1 B 1 Set up reaction Run Duplex PCR A 2 B 2 A 30 CYCLE 28. 3. 2017 B 15

Materials and Methods Samples were collected from commercial poultry flocks in central region Iran (Qom (Central province, Kashan, and Esfahan). The Most of the samples were obtained from flocks with clinical signs of a probably infection by Mycoplasma spp (MG, MS). Taking 50 samples included air sac, choanal -cleft and tracheal swabs from flocks. Then aliquot of 0. 5 ml was removed to a 1. 5 ml tube for the DNA extraction. After filtering by 0. 45 filters, 0. 3 ml sample was taken for broth culture, PPLO broth and PPLO agar medium. Broth and agar media were incubated under micro aerophilic condition with 98% relative humidity and checked for color changes of broth two passages. 28. 3. 2017 16

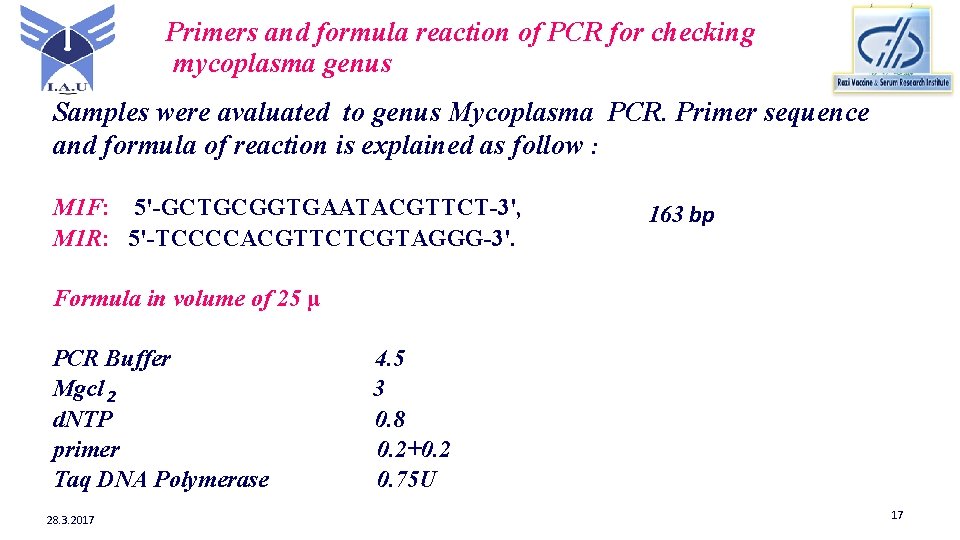
Primers and formula reaction of PCR for checking mycoplasma genus Samples were avaluated to genus Mycoplasma PCR. Primer sequence and formula of reaction is explained as follow : M 1 F: 5'-GCTGCGGTGAATACGTTCT-3', M 1 R: 5'-TCCCCACGTTCTCGTAGGG-3'. 163 bp Formula in volume of 25 µ PCR Buffer Mgcl 2 d. NTP primer Taq DNA Polymerase 28. 3. 2017 4. 5 3 0. 8 0. 2+0. 2 0. 75 U 17

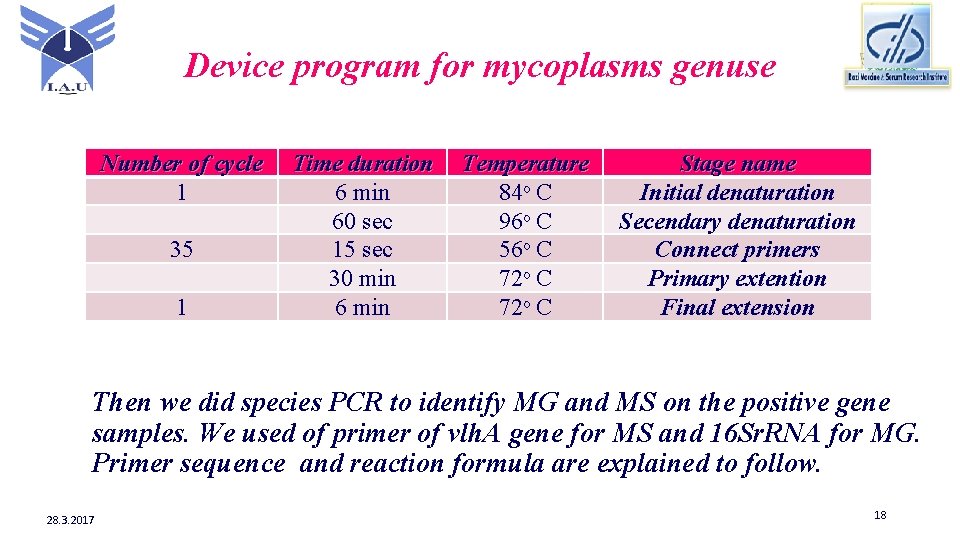
Device program for mycoplasms genuse Number of cycle 1 35 1 Time duration 6 min 60 sec 15 sec 30 min 6 min Temperature 84ᵒ C 96ᵒ C 56ᵒ C 72ᵒ C Stage name Initial denaturation Secendary denaturation Connect primers Primary extention Final extension Then we did species PCR to identify MG and MS on the positive gene samples. We used of primer of vlh. A gene for MS and 16 Sr. RNA for MG. Primer sequence and reaction formula are explained to follow. 28. 3. 2017 18

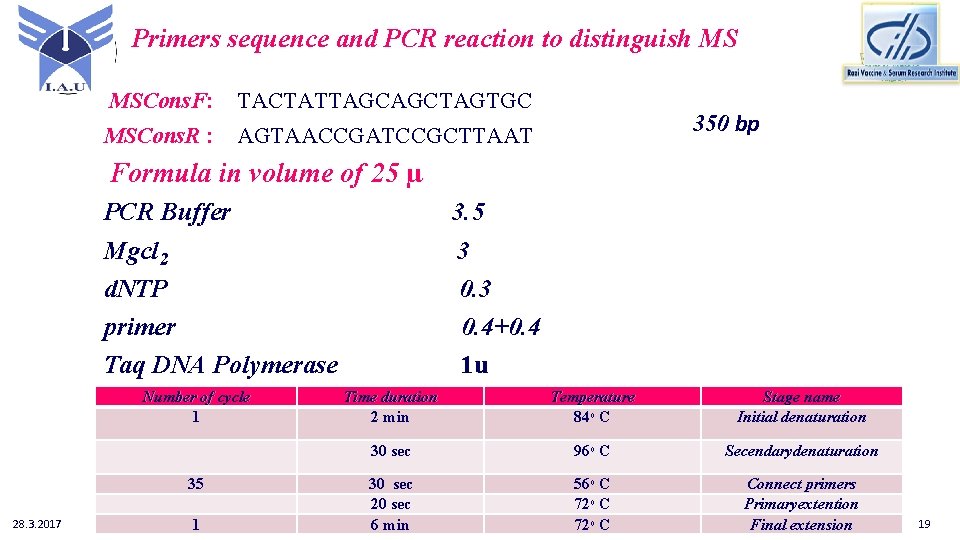
Primers sequence and PCR reaction to distinguish MS MSCons. F: TACTATTAGCAGCTAGTGC MSCons. R : AGTAACCGATCCGCTTAAT 350 bp Formula in volume of 25 µ PCR Buffer Mgcl 2 d. NTP primer Taq DNA Polymerase Number of cycle 1 35 28. 3. 2017 1 3. 5 3 0. 4+0. 4 1 u Time duration 2 min Temperature 84ᵒ C Stage name Initial denaturation 30 sec 96ᵒ C Secendarydenaturation 30 sec 20 sec 6 min 56ᵒ C 72ᵒ C Connect primers Primaryextention Final extension 19

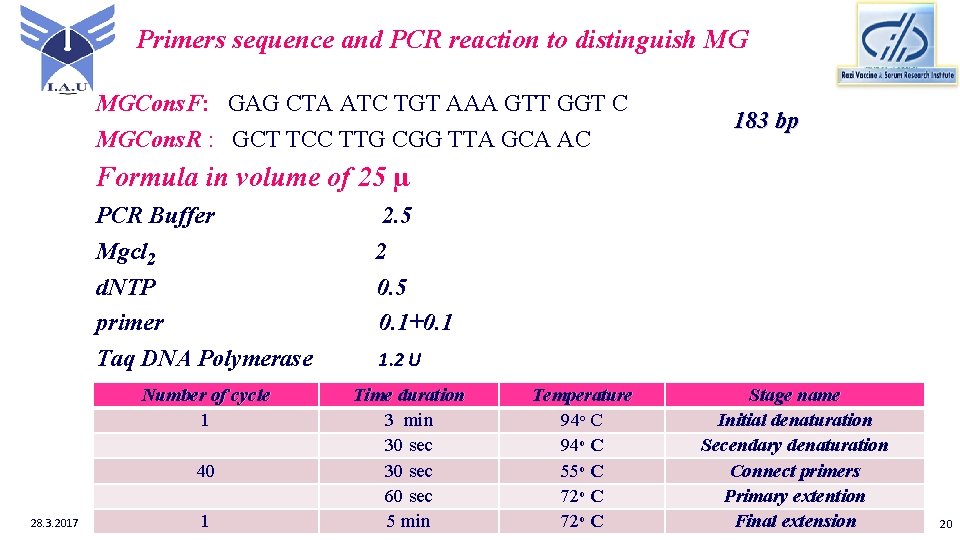
Primers sequence and PCR reaction to distinguish MG MGCons. F: GAG CTA ATC TGT AAA GTT GGT C MGCons. R : GCT TCC TTG CGG TTA GCA AC 183 bp Formula in volume of 25 µ PCR Buffer Mgcl 2 d. NTP primer Taq DNA Polymerase 2. 5 2 0. 5 0. 1+0. 1 Number of cycle 1 Time duration 3 min 30 sec 60 sec 5 min 40 28. 3. 2017 1 1. 2 U Temperature 94ᵒ C 55ᵒ C 72ᵒ C Stage name Initial denaturation Secendary denaturation Connect primers Primary extention Final extension 20

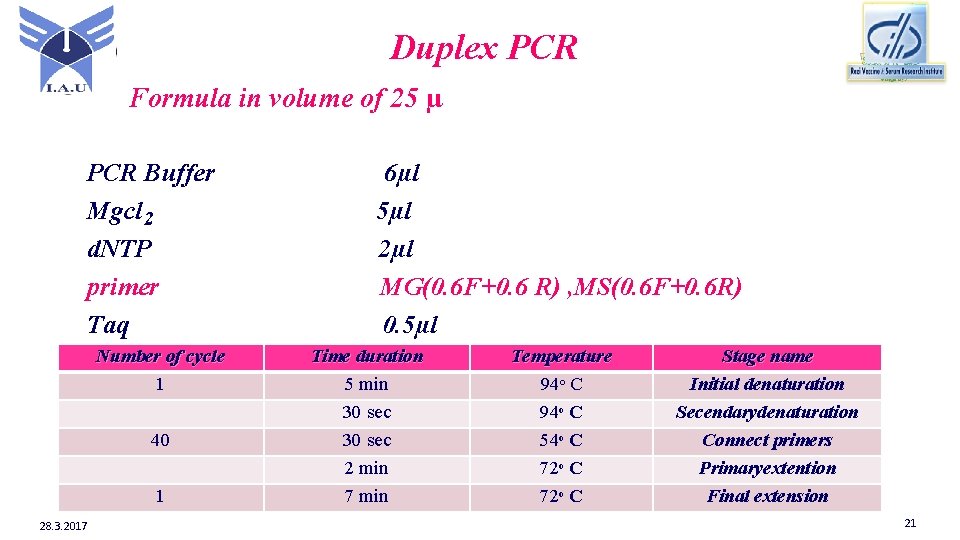
Duplex PCR Formula in volume of 25 µ PCR Buffer Mgcl 2 d. NTP primer Taq Number of cycle 1 40 1 28. 3. 2017 6µl 5µl 2µl MG(0. 6 F+0. 6 R) , MS(0. 6 F+0. 6 R) 0. 5µl Time duration 5 min 30 sec 2 min 7 min Temperature 94ᵒ C 54ᵒ C 72ᵒ C Stage name Initial denaturation Secendarydenaturation Connect primers Primaryextention Final extension 21

Results After sampling (50 samples), DNA was extracted with phenol chloroform method, then genes PCR reaction was conducted. we did single PCR for identification MG species and MS species on 20 samples that were positive in genes PCR. Among them 7 samples were MS positive, 7 samples were MG positive and 3 samples were negative. Where as Duplex PCR for distingushing MG and MS was conducted for first time. So at the first we setup it with positive control. Then other samples were checked with this technique. 28. 3. 2017 22

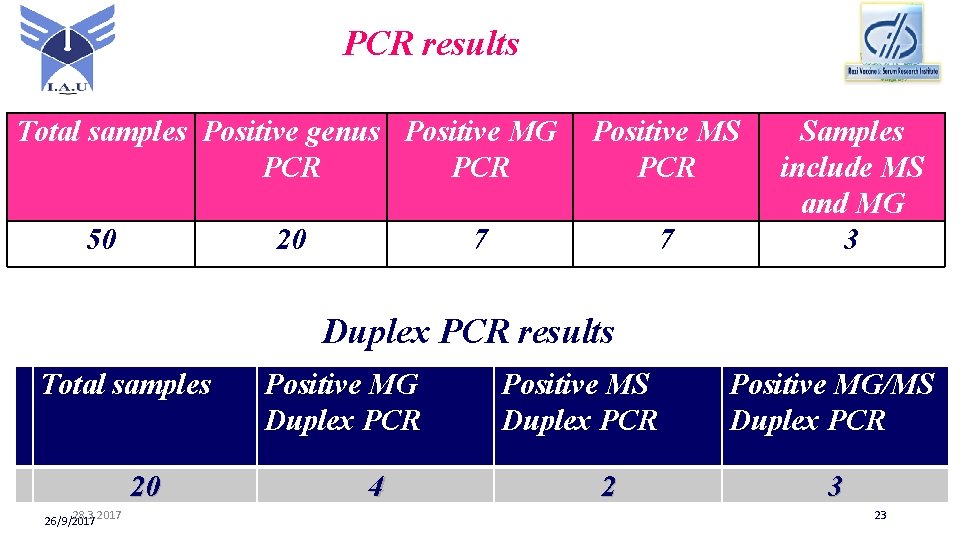
PCR results Total samples Positive genus Positive MG PCR 50 20 Positive MS PCR 7 7 Samples include MS and MG 3 Duplex PCR results Total samples 20 28. 3. 2017 26/9/2017 Positive MG Duplex PCR 4 Positive MS Duplex PCR 2 Positive MG/MS Duplex PCR 3 23

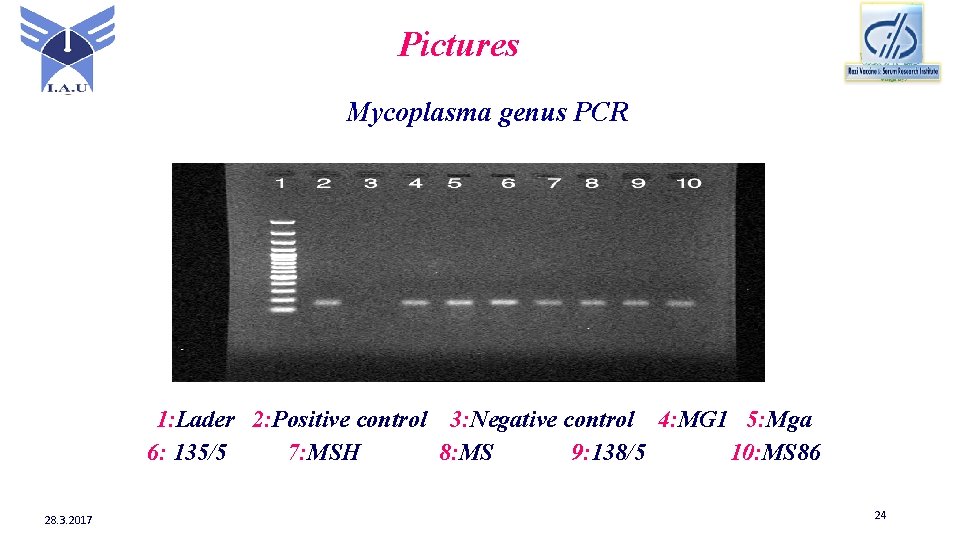
Pictures Mycoplasma genus PCR 1: Lader 2: Positive control 3: Negative control 4: MG 1 5: Mga 6: 135/5 7: MSH 8: MS 9: 138/5 10: MS 86 28. 3. 2017 24

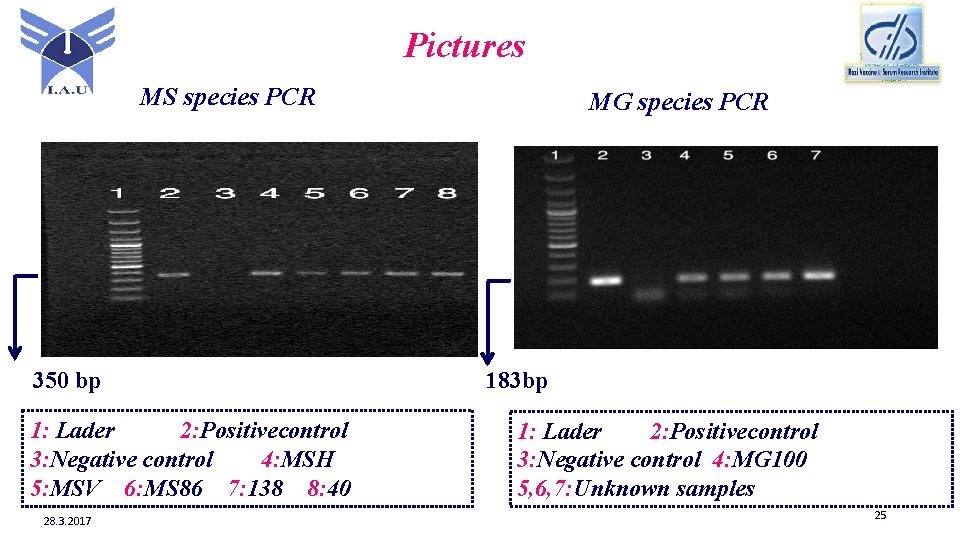
Pictures MS species PCR 350 bp 1: Lader 2: Positivecontrol 3: Negative control 4: MSH 5: MSV 6: MS 86 7: 138 8: 40 28. 3. 2017 MG species PCR 183 bp 1: Lader 2: Positivecontrol 3: Negative control 4: MG 100 5, 6, 7: Unknown samples 25

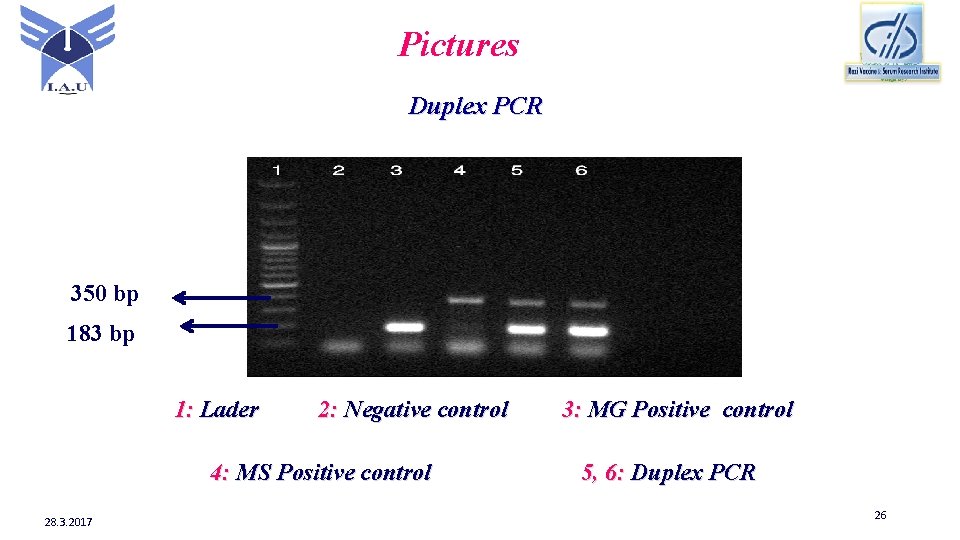
Pictures Duplex PCR 350 bp 183 bp 1: Lader 2: Negative control 4: MS Positive control 28. 3. 2017 3: MG Positive control 5, 6: Duplex PCR 26

Discussion Nowadays in addition to PCR, different methods use for identification microorganisms Kathleen. A. Stellrecht (2003), used of Multiplex PCR to identify Ureaplasma, Mycoplasma genitalium and Mycoplasma hominise. A. V. Sprigin (2010) , by Duplex REAL-TIME Taqman PCR checked MG and MS in clinical samples. In this study we could do simultaneously identify MG and MS by Duplex PCR assay. we used 16 Sr. RNA for identification Mycoplasma genus and MG. Also we used vlh. A gene to distinguish MS. All of samples (field and positive samples) were identified. duplex PCR is more rapid and inexpensive method than the single PCR for detection of MG & MS. 28. 3. 2017 27

Conclusion The results of this study showed that Duplex PCR is faster and inexpensive method than the single PCR for detection of MG & MS. So Duplex PCR can be an alternative method for PCR to detect MG & MS with each other. In the present study Duplex PCR is more capable to identify MG than MS and bands of MG are stronger than bands of MS. This study suggested for better identification of MS, it could be changed in program or master mix. 28. 3. 2017 28

Summary Mycoplasma gallisepticum (M. G) and Mycoplasma synoviae (M. S) have been recognized as common respiratory pathogens especially in chickens causing lots economical losses in poultry industries. The aim of this study was to develop and validate Duplex Polymerase Chain Reaction (Duplex PCR) for simultaneous detection of MG & MS. The research was conducted in Mycoplasma Reference Laboratory, Razi Vaccine and Serum Research Institute, Karaj, Iran. A total of 50 samples from tracheas, lungs and air sacs were taken from commercial broiler chicken farms in Iran. The samples were cultured in PPLO broth supplemented for M. S and M. G isolation and bacteria DNA were extracted by phenol/chloroform extraction method. 28. 3. 2017 29

Summary The conserved region of 16 Sr. RNA gene which was applied for the detection of Mycoplasma genus in 163 bp fragment and MG in 183 bp fragment and vlh. A gene was also employed for detection of MS in 350 bp fragment. Hence, Duplex PCR amplified the conserved region of 16 Sr. RNA and vlh. A gene which were applied for detection of MG &MS. 20 samples in Mycoplasma genus, and 7 samples in MG & MS were positive in the single PCR whereas in 3 samples of MG & MS were simultaneously positive in the Duplex PCR method. The results showed that Duplex PCR was successful to identify MG & MS at the same time and suggested that duplex PCR is more rapid and inexpensive method than the single PCR for detection of MG & MS. 28. 3. 2017 30

Thanks for your attention 28. 3. 2017 31
![Reference 1 Bardbury J M Kleven S H 2003 Mycoplasma Iowa Infection in disease Reference [1] Bardbury J. M; Kleven, S. H. (2003). Mycoplasma Iowa Infection. in: disease](https://slidetodoc.com/presentation_image_h2/8942402624bfb89207674d031871603a/image-32.jpg)
Reference [1] Bardbury J. M; Kleven, S. H. (2003). Mycoplasma Iowa Infection. in: disease of poultry. 11 th edition. Saif. Y. M et all. Iowa State University Press. P: 766 -772. [2] Bercic R. L; Slavec, B; Lavric, M; Narat, M; Bidovec, A; Dovc, Peter; Benecina, D. (2007). Identification of major hmmunogenic proteins of mycoplasma synoviae isolates. Veterinary microbiology. Preprint. [3] Carnaghan, R. B. A. 1961. Egg transmission of infectious synovitis. J Comp Pathol 71: 279 -285. [4] Collett, S. R. (2005). Monitoring broiler breeder flocks for Mycoplasma gallisepticum infection after vaccination with ts- 11. Department of production animal studies, faculty of veterinary science, university of Pretoria, pp: 1 -79. [5] Dowling, L. (1984). Toxicity of Ionophores. Vet Rec. 114 -128. [6] Dohms, J. E. , Hnatow, L. L. , Whetzel, P. Morgan, R. , and Keeler, C. L. Jr. (1993). Identification of the putative cytadhesion gene of Mycoplasma gallisepticum and its use as a DNA probe. Avian Diseases 37: 380 -388. [7] Fan, H. H. , Kleven, S. H and Jackwood, M. W. (1995). Application of polymerase chain reaction with arbitrary primers to strain identification of Mycoplasma gallisepticum. Avian Diseases 39: 729 -735. [8] Fan, H. H. , Kleven, S. H. , Jachwood, M. W. , Johansson K. E. , Pettersson, B. , and Levisohn, S. (1995). Species identification of avian Mycoplasma by polymerase chain reaction and restriction fragment length polymorphism analysis. Avian Diseases 39: 398 -407 [8] Garia , M. , Jachwood M. W. , Levisohn, S. , Kleven, S. H. (1999). Use of specific-specefic oligoucleotid probe to detect Mycoplasma gallisepticum, M. synoviae, and M. iowa PCR amplitication products. J. Vet. Diag. Invest. 8(1), p: 56 -63 [9] Ley, D. H (2008). Mycoplasma gallisepticum infection. In: Saif Y. M. , Barnes H. J. , Glisson J. R, Fadly A. M. , Mc. Dougald L. R. & D. E, Swayne eds. Disease of poultry, 12 th Edition, Iowa State University Press, lowa, USA, pp: 808 -834 [10] Liu, T. , M. Garcia, S. Levisohn, D. Yogev, and S. H. Kleven. (2001). Molecular variability of theuu adhesion-encoding gene pvp. A among Mycoplasma gallisepticum strains and its application in diagnosis. Journal of Clinical Microbiology 39: 1882 -1888. [11] Lysnyansky, I. , M. Garcia, and S. Levisohn. (2005). Use of mgc 2 -polymerase chain reaction-restriction fragment length polymorphism for rapid differentiation between field isolates and vaccine strains of Mycoplasma gallisepticum in Israel. Avian Diseases 49: 238 -245. 28. 3. 2017 32
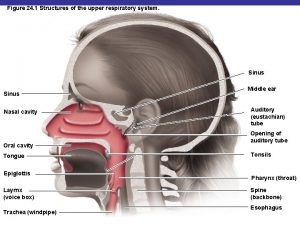
 Mycoplasma pneumoniae
Mycoplasma pneumoniae Mycoplasma pneumoniae
Mycoplasma pneumoniae Dienes stain
Dienes stain Vagninitis
Vagninitis Presumptive identification vs positive identification
Presumptive identification vs positive identification Title fly of a report
Title fly of a report Title title
Title title Solve the simultaneous equations
Solve the simultaneous equations Kds micronex
Kds micronex Simultaneous heat and mass transfer
Simultaneous heat and mass transfer Simultaneous integration example
Simultaneous integration example Penyelesaian persamaan simultan
Penyelesaian persamaan simultan Quadratic and linear simultaneous equations
Quadratic and linear simultaneous equations Non linear simultaneous equations
Non linear simultaneous equations 7 elements of music
7 elements of music Simultaneous linear equations problems
Simultaneous linear equations problems Linear simultaneous equations
Linear simultaneous equations Simultaneous multithreading
Simultaneous multithreading Simultaneous equations step by step
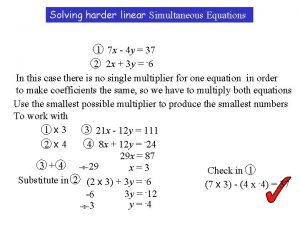
Simultaneous equations step by step Elimination method
Elimination method Elimination method simultaneous equations
Elimination method simultaneous equations Simultaneous equations
Simultaneous equations Elimination method examples
Elimination method examples Persamaan linear
Persamaan linear What is simultaneous evacuation
What is simultaneous evacuation Simultaneous extinction
Simultaneous extinction Simultaneous extinction
Simultaneous extinction Simultaneous access in network
Simultaneous access in network Dr frost simultaneous equations
Dr frost simultaneous equations Simultaneous interpreting definition
Simultaneous interpreting definition Simultaneous equations
Simultaneous equations Simultaneous interpretation in turkey
Simultaneous interpretation in turkey What is force summation
What is force summation