Timedependent densityfunctional description of the 1 La state

- Slides: 19

Time-dependent density-functional description of the 1 La state in polycyclic aromatic hydrocarbons: Charge-transfer character in disguise? Ryan M. Richard 66 th International Molecular Spectroscopy Symposium June 24, 2011

Background Studies* show traditional TD-DFT has problems with some polycyclic aromatic hydrocarbons (PAHs) Particular focus on 1ππ* states 1 La and 1 Lb States differ by: Polarization Excitation character *Grimme, S. ; Parac, M. Chem. Phys. Chem 2003, 4, 292. Parac, M. ; Grimme, S. Chem. Phys. 2003, 292, 11. Wong, B. M. ; Hsieh, T. H. JCTC. 2010, 6, 3704.

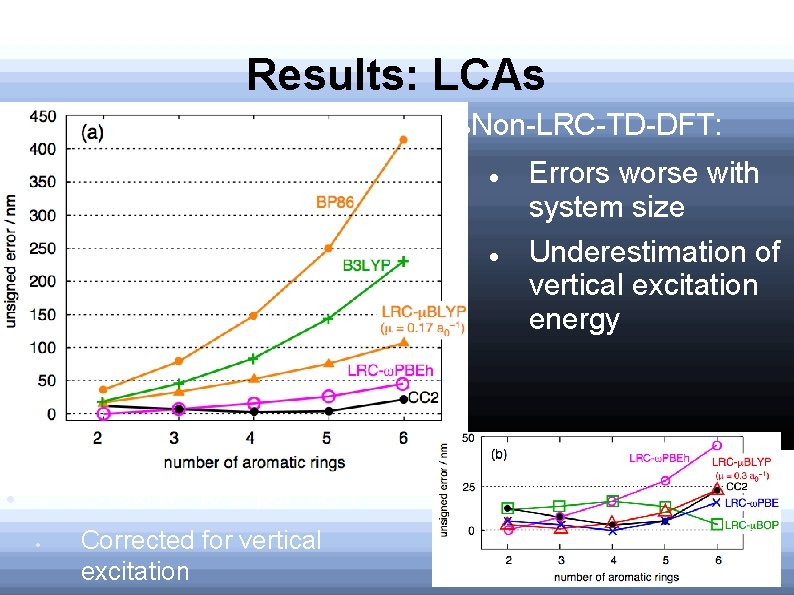
Results: LCAs Non-LRC-TD-DFT: Errors relative to experiment Corrected for vertical excitation Errors worse with system size Underestimation of vertical excitation energy

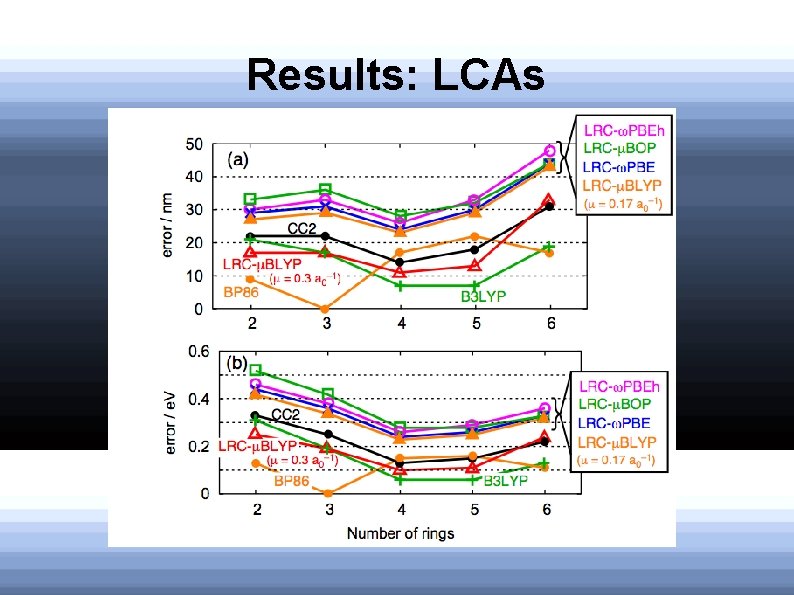
Results: LCAs

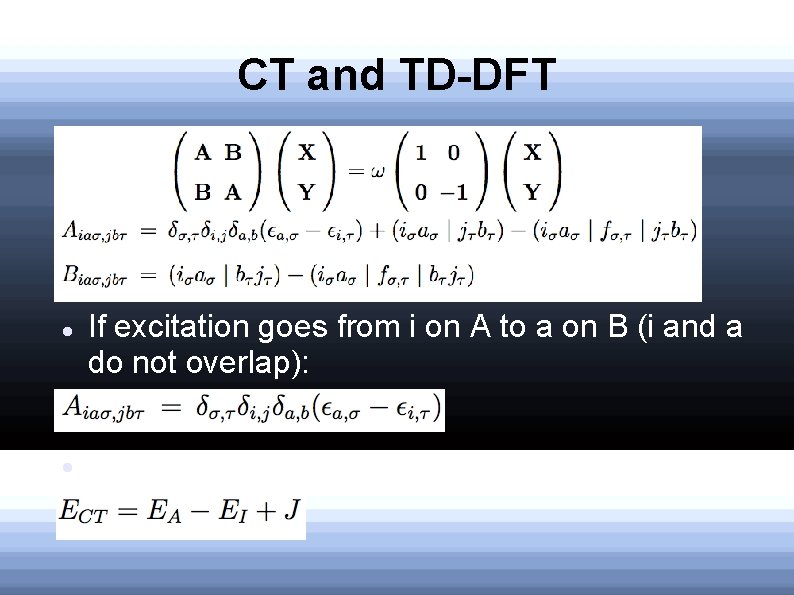
CT and TD-DFT If excitation goes from i on A to a on B (i and a do not overlap): The proper CT energy is:

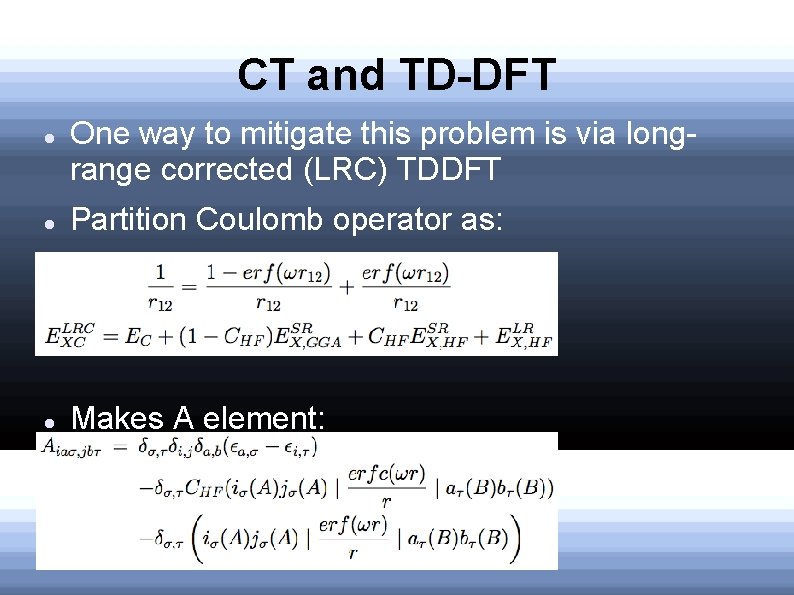
CT and TD-DFT One way to mitigate this problem is via longrange corrected (LRC) TDDFT Partition Coulomb operator as: Makes A element:

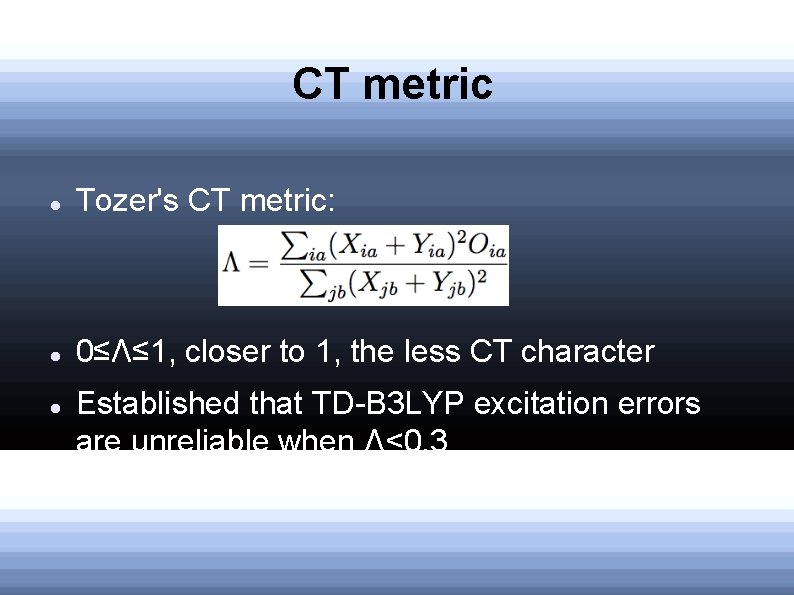
CT metric Tozer's CT metric: 0≤Λ≤ 1, closer to 1, the less CT character Established that TD-B 3 LYP excitation errors are unreliable when Λ<0. 3

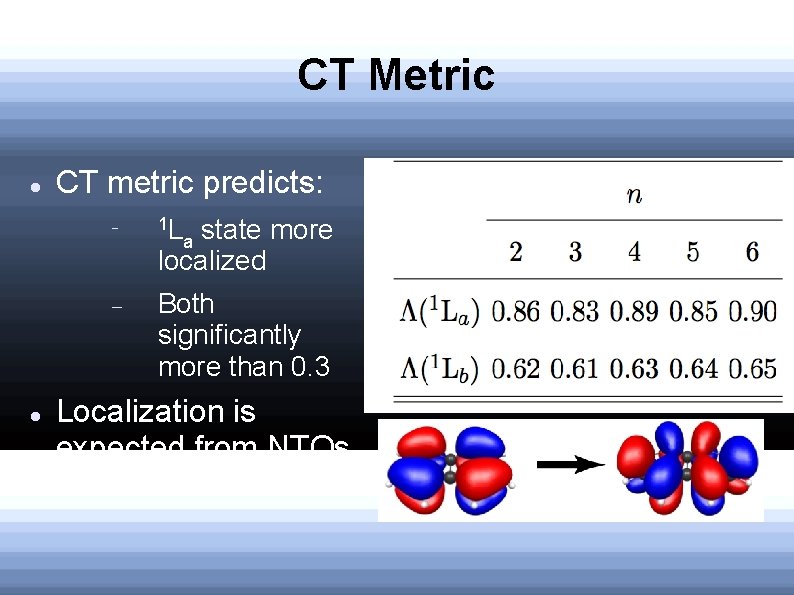
CT Metric CT metric predicts: 1 L Both significantly more than 0. 3 state more localized a Localization is expected from NTOs

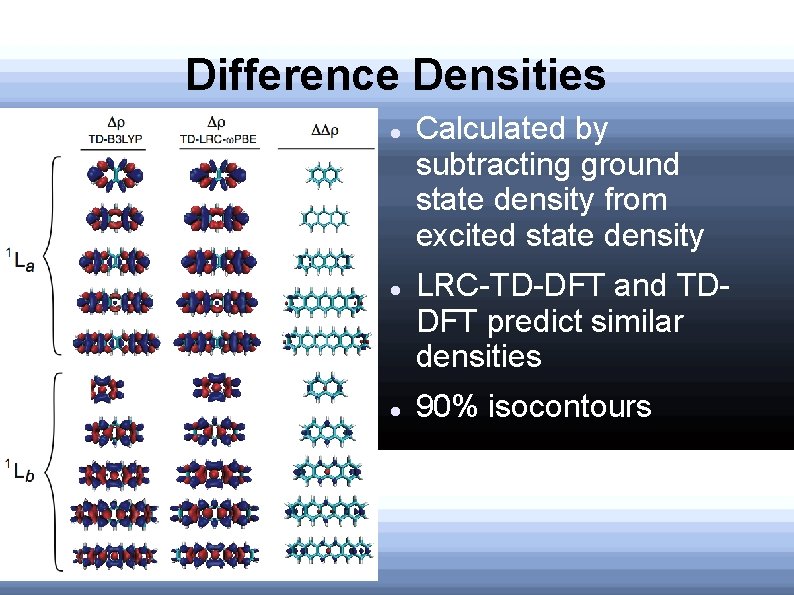
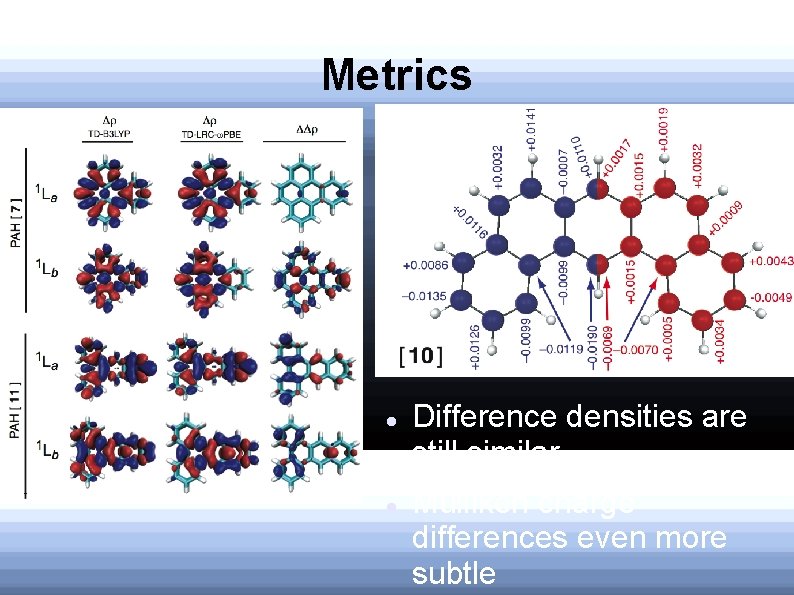
Difference Densities Calculated by subtracting ground state density from excited state density LRC-TD-DFT and TDDFT predict similar densities 90% isocontours

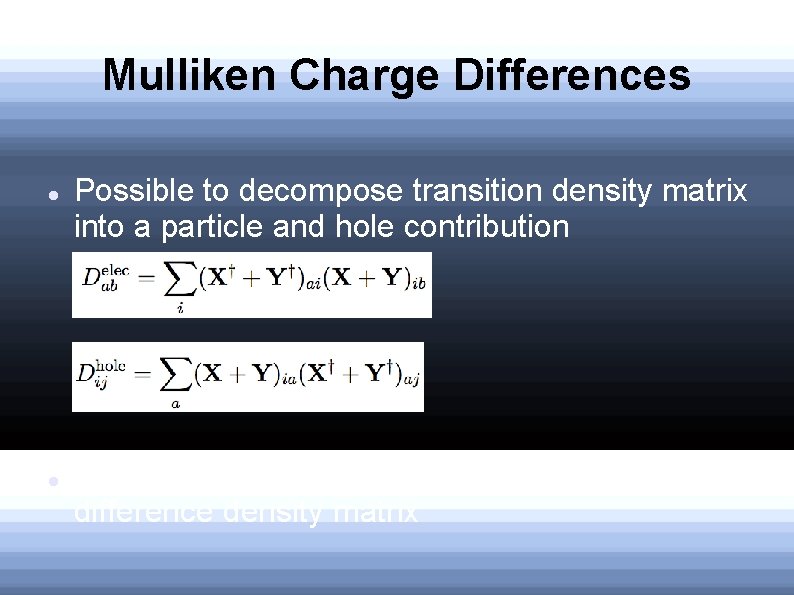
Mulliken Charge Differences Possible to decompose transition density matrix into a particle and hole contribution Sum of these two densities is the 1 electron difference density matrix

Mulliken Charge Differences If the electron and hole densities are in the atomic orbital basis: By restricting the summation to AOs on a given atom, this is analogous to a Mulliken charge for the given atom Can also be done for Löwdin charges

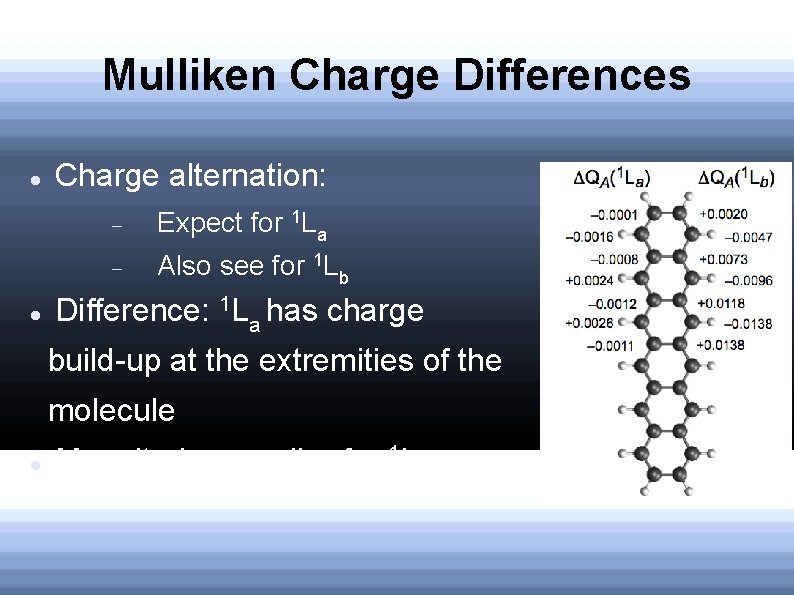
Mulliken Charge Differences Charge alternation: Expect for 1 La Also see for 1 Lb Difference: 1 La has charge build-up at the extremities of the molecule Magnitudes smaller for 1 La

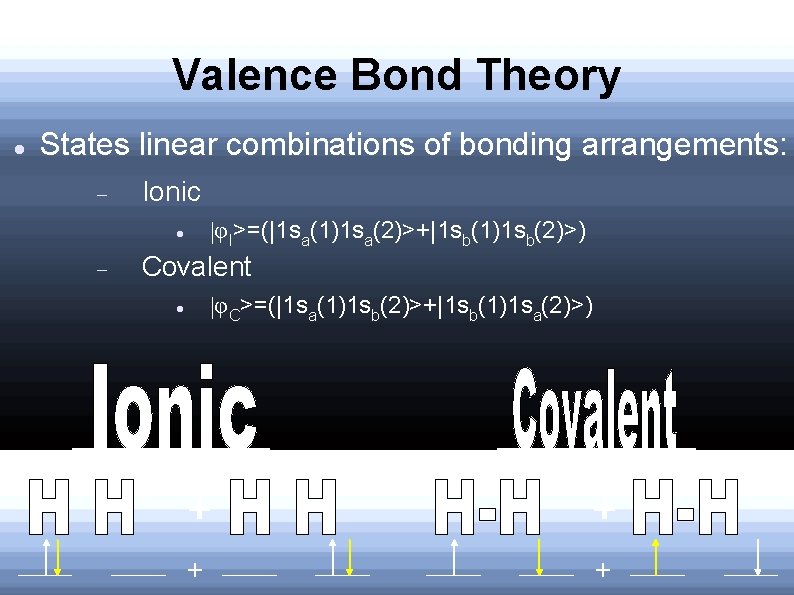
Valence Bond Theory States linear combinations of bonding arrangements: Ionic φI>=(|1 sa(1)1 sa(2)>+|1 sb(1)1 sb(2)>) Covalent φC>=(|1 sa(1)1 sb(2)>+|1 sb(1)1 sa(2)>) - + +

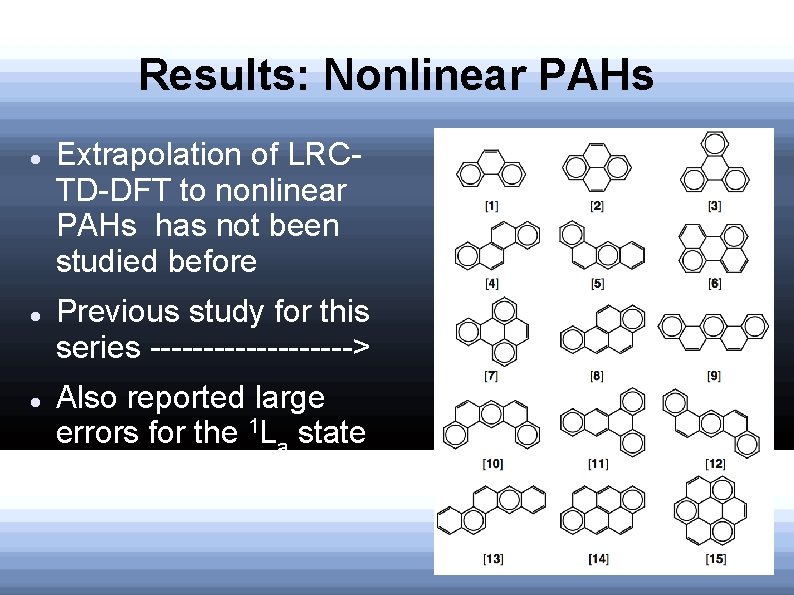
Results: Nonlinear PAHs Extrapolation of LRCTD-DFT to nonlinear PAHs has not been studied before Previous study for this series ----------> Also reported large errors for the 1 La state

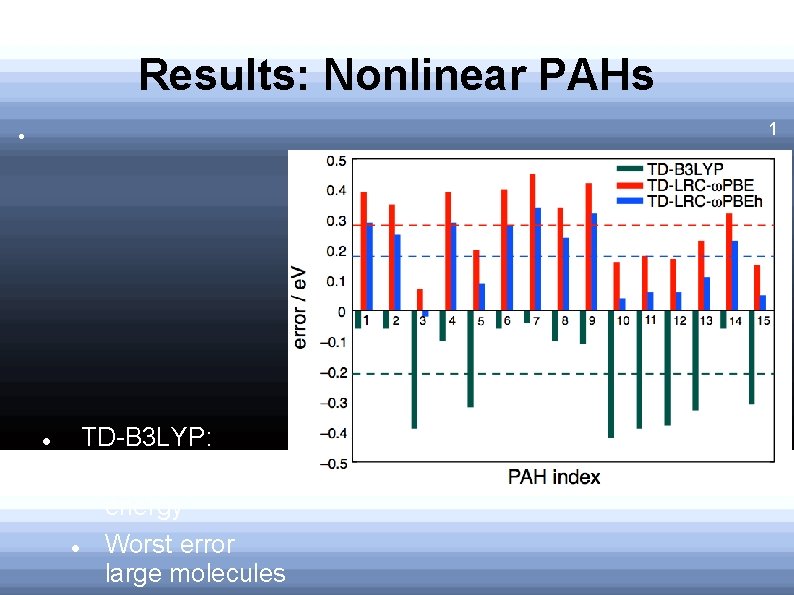
Results: Nonlinear PAHs 1 L a s t a t e : TD-B 3 LYP: Underestimates energy Worst error large molecules

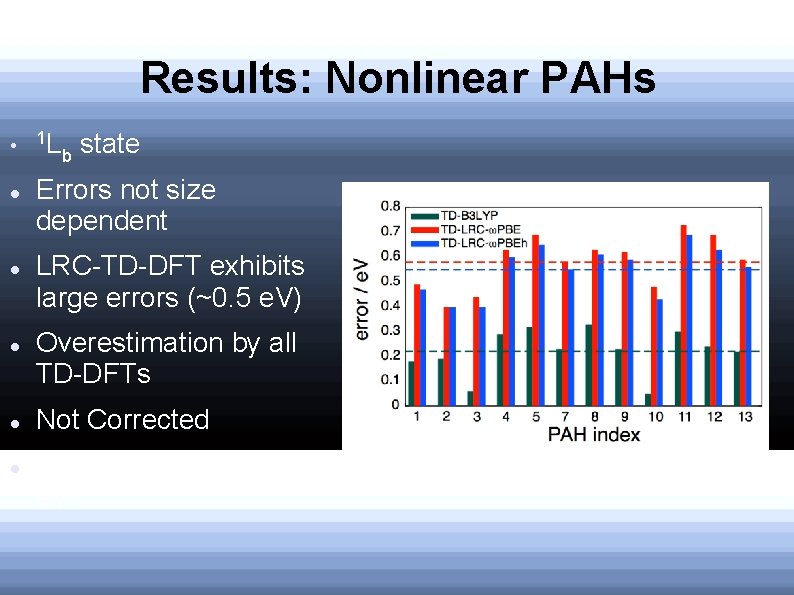
Results: Nonlinear PAHs 1 L b state Errors not size dependent LRC-TD-DFT exhibits large errors (~0. 5 e. V) Overestimation by all TD-DFTs Not Corrected Suggested to be ~0. 03 e. V

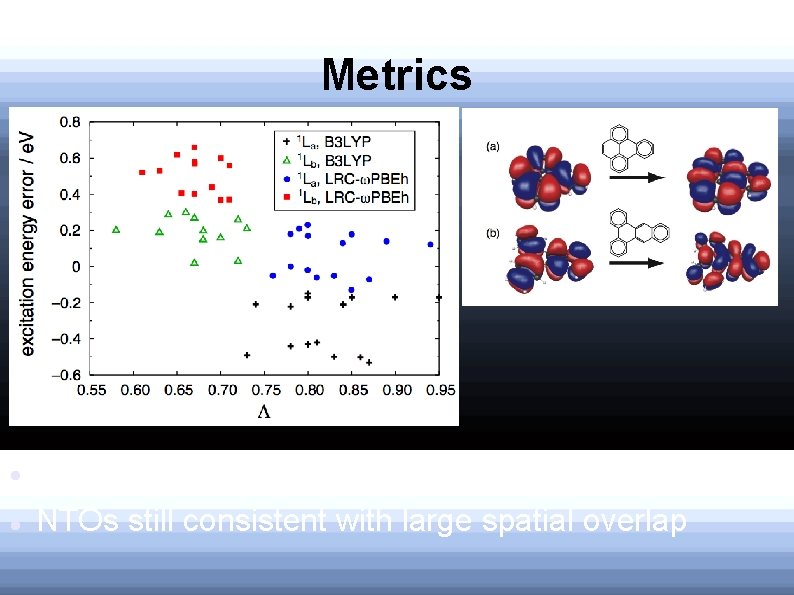
Metrics CT metric still fails to predict CT character NTOs still consistent with large spatial overlap

Metrics Difference densities are still similar Mulliken charge differences even more subtle

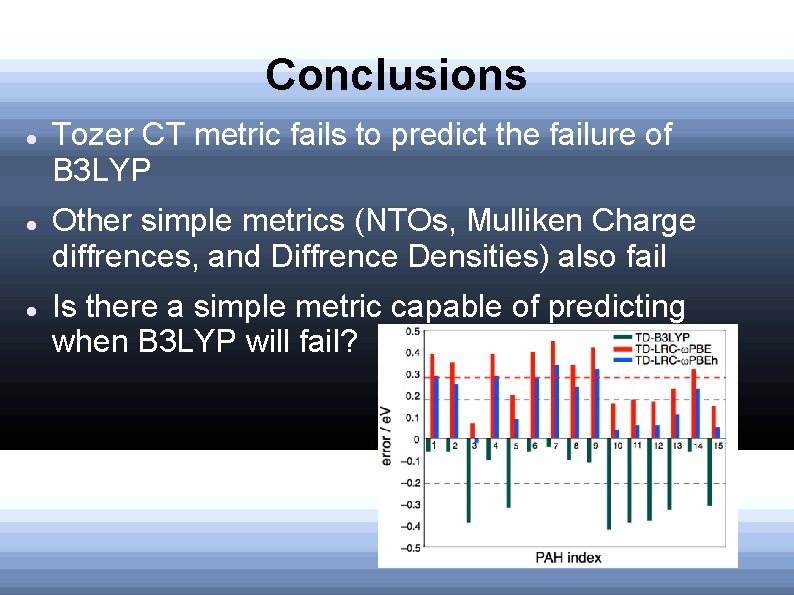
Conclusions Tozer CT metric fails to predict the failure of B 3 LYP Other simple metrics (NTOs, Mulliken Charge diffrences, and Diffrence Densities) also fail Is there a simple metric capable of predicting when B 3 LYP will fail?