Thomson Model The plum pudding J J Thomson







- Slides: 23

Thomson Model – The ‘plum pudding’

J. J. Thomson • 1897 – Joseph Thomson used the cathode-ray tube and discovered the electron.

Rutherford model

Ernest Rutherford (1871 -1937) • Learned physics in J. J. Thomson’ lab. • Noticed that ‘alpha’ particles were sometime deflected by something in the air. • Gold-foil experiment

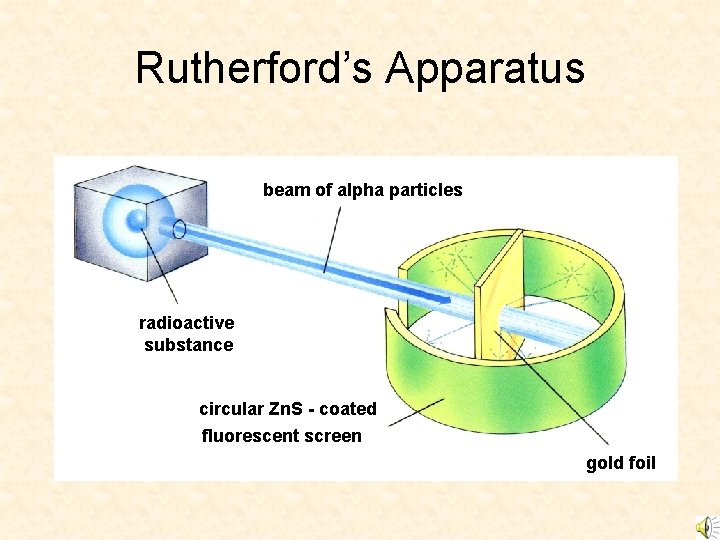
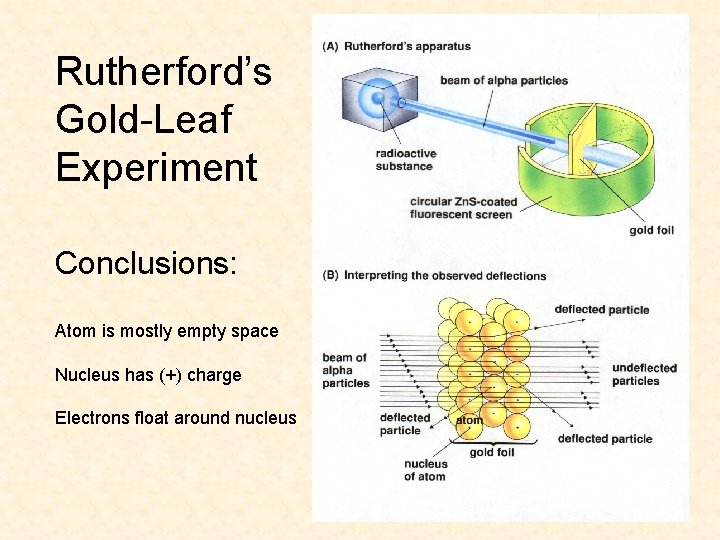
Rutherford’s Apparatus beam of alpha particles radioactive substance circular Zn. S - coated fluorescent screen gold foil

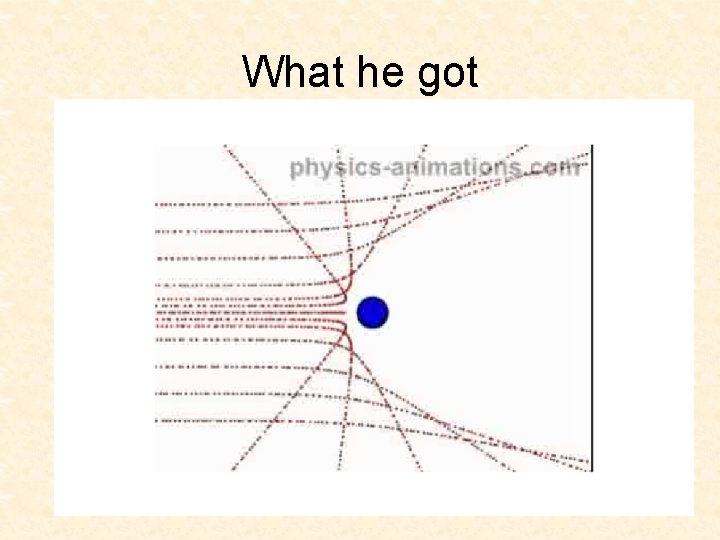
Rutherford ‘Scattering’ • In 1909 Rutherford undertook a series of experiments • He fired a (alpha) particles at a very thin sample of gold foil • According to the Thomson model the a particles would only be slightly deflected • Rutherford discovered that they were deflected through large angles and could even be reflected straight back to the source

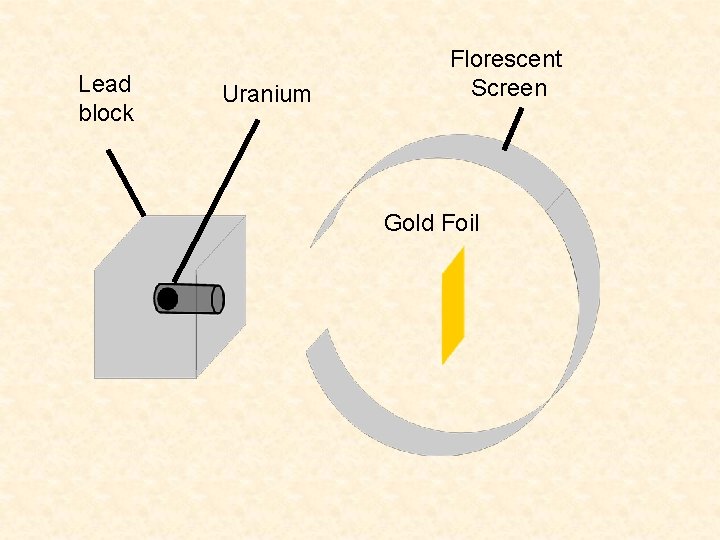
Lead block Uranium Florescent Screen Gold Foil

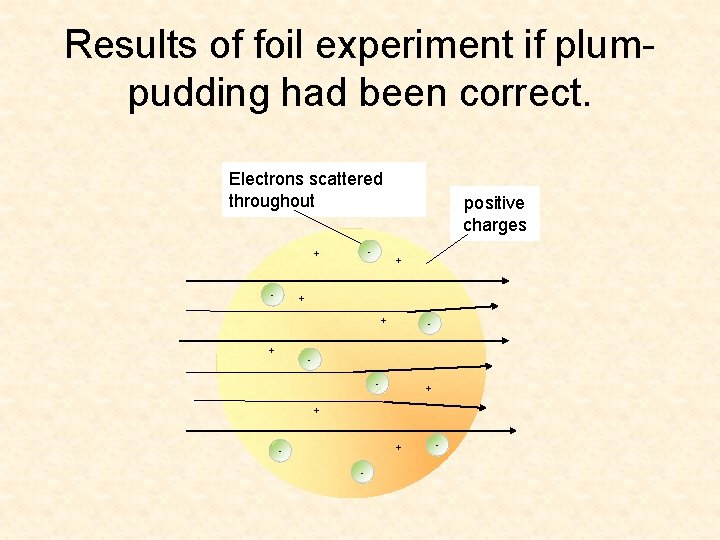
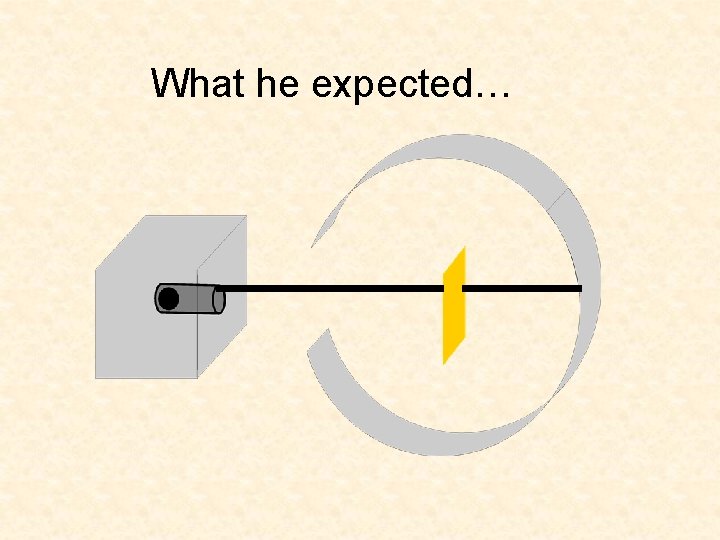
What He Expected • The alpha particles to pass through without changing direction (very much) • Because …. . • The positive charges were spread out evenly. Alone they were not enough to stop the alpha particles

Results of foil experiment if plumpudding had been correct. Electrons scattered throughout - + - positive charges + + - - + + + - -

What he expected…

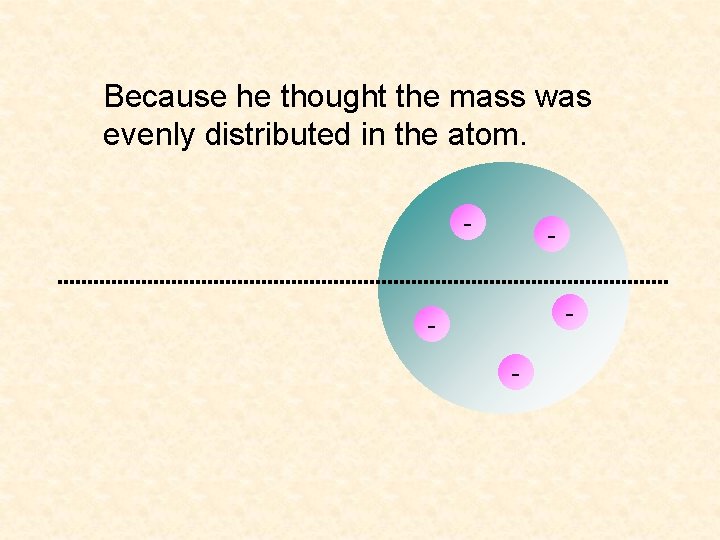
Because he thought the mass was evenly distributed in the atom. - - -

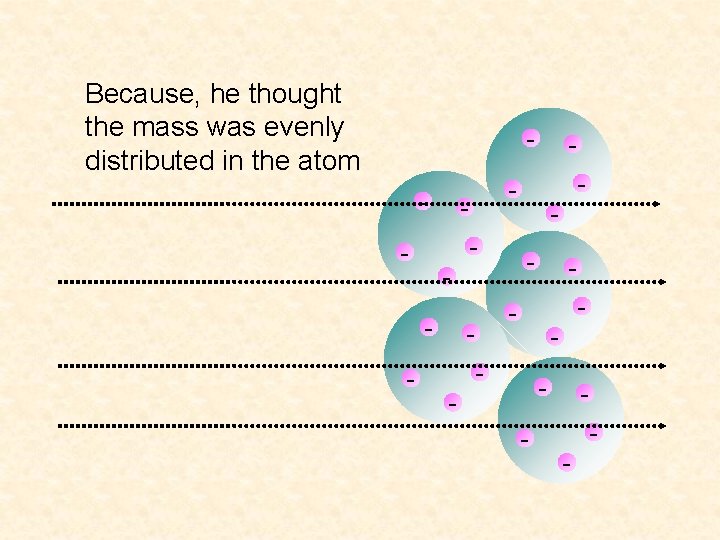
Because, he thought the mass was evenly distributed in the atom - - - - -

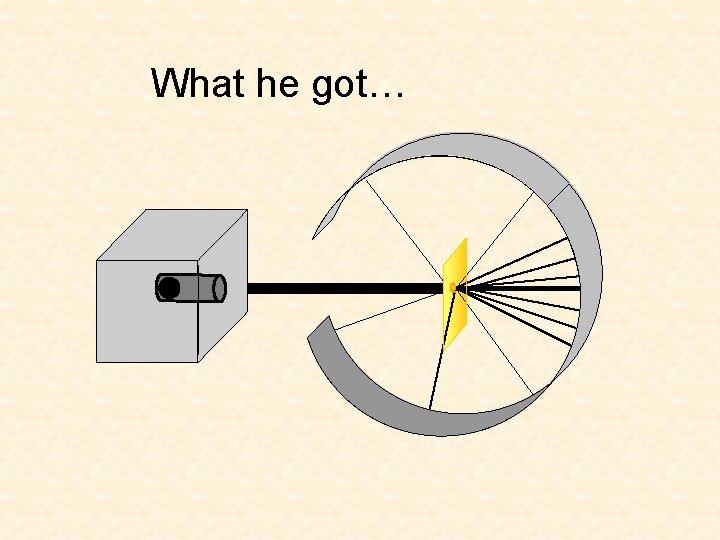
What he got

What he got…

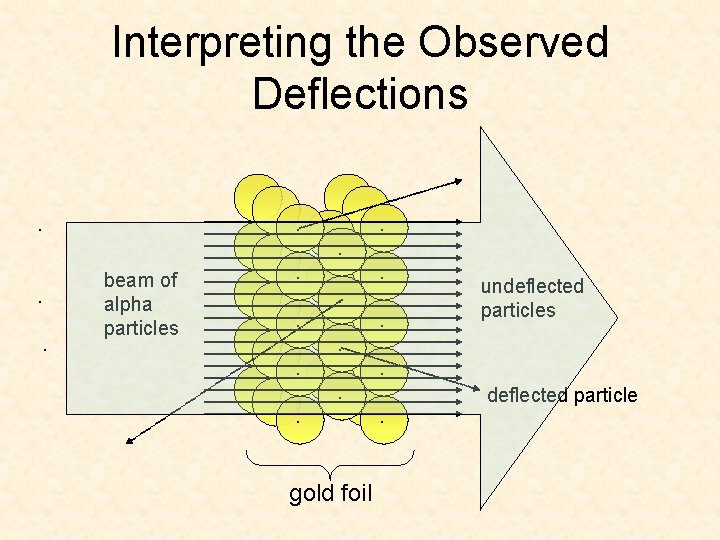
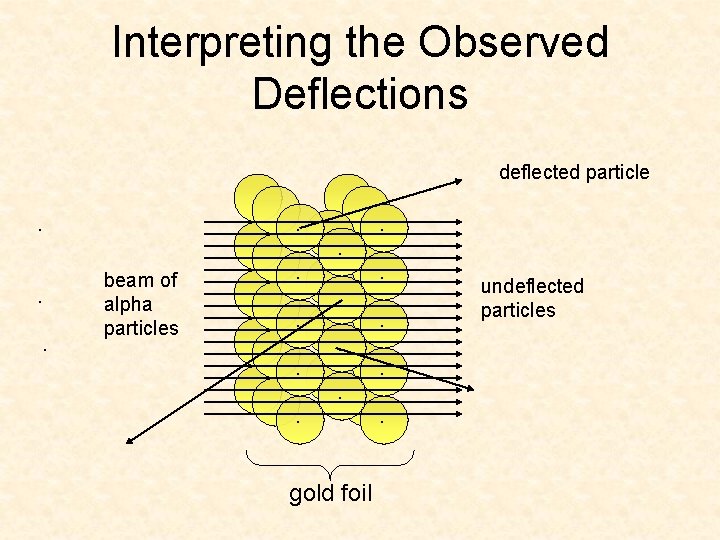
Interpreting the Observed Deflections. . beam of alpha particles . . . . gold foil . . . undeflected particles . . deflected particle

Density and the Atom • Since most of the particles went through, the atom was mostly empty. • Because the alpha rays were deflected so much, the positive pieces it was striking were heavy. • Small volume and big mass = big density • This small dense positive area is the nucleus

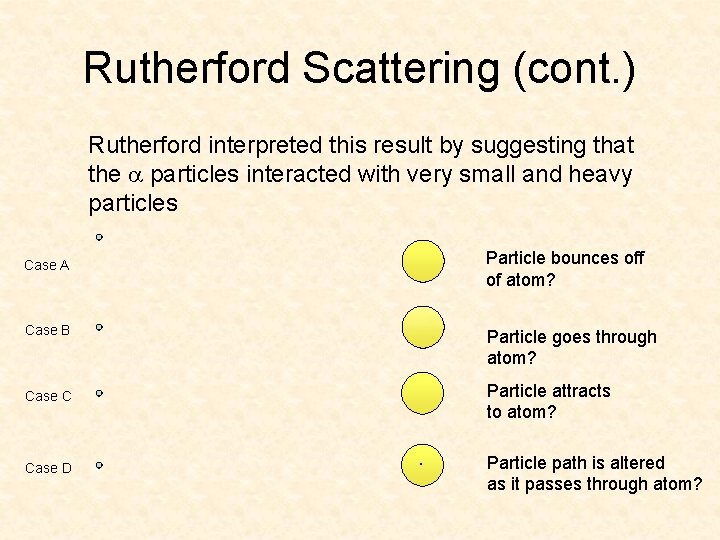
Rutherford Scattering (cont. ) Rutherford interpreted this result by suggesting that the a particles interacted with very small and heavy particles Particle bounces off of atom? Case A Case B Particle goes through atom? Particle attracts to atom? Case C Case D . Particle path is altered as it passes through atom?

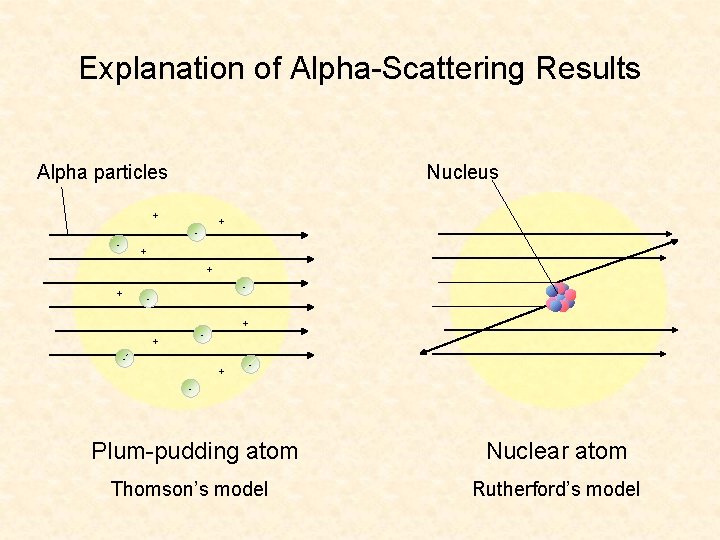
Explanation of Alpha-Scattering Results Alpha particles Nucleus + + - - + + - + - - Plum-pudding atom Nuclear atom Thomson’s model Rutherford’s model

Interpreting the Observed Deflections deflected particle . . beam of alpha particles . . . . gold foil . . . undeflected particles

Rutherford’s Gold-Leaf Experiment Conclusions: Atom is mostly empty space Nucleus has (+) charge Electrons float around nucleus

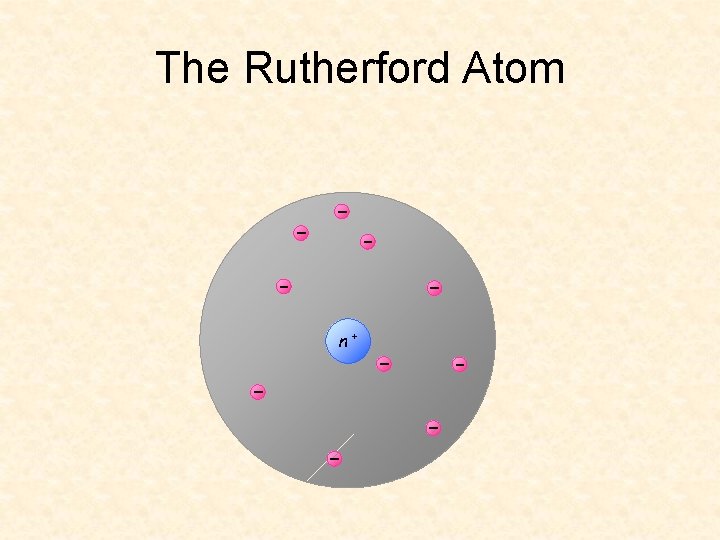
The Rutherford Atom n+

Summary of the Rutherford experiment

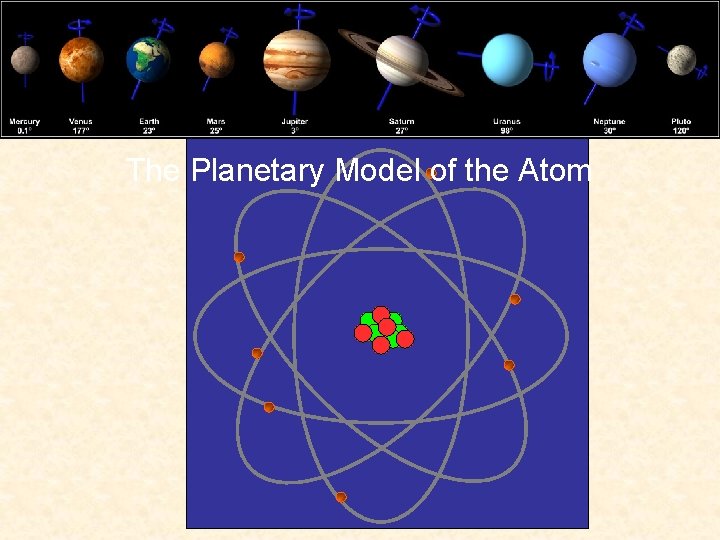
Bohr Atom The Planetary Model of the Atom