J J Thomson The Plum Pudding or Raisin




- Slides: 8

J. J. Thomson The Plum Pudding or Raisin Bun Model

The Problem… • Dalton Model – Matter consists of tiny particles, atoms – The Particles are indestructible – Atoms of each element have a different mass – Atoms react and combine in simple whole number ratios Atoms are one indivisible sphere.

The Design… • Cathode ray tube • Electrometer • Magnets

The Experiment & Evidence • Actually there were three 1) Using the cathode ray tube and the electrometer, no charge was indicated unless the ray was deflected towards the electrometer The charge is a property of the ray.

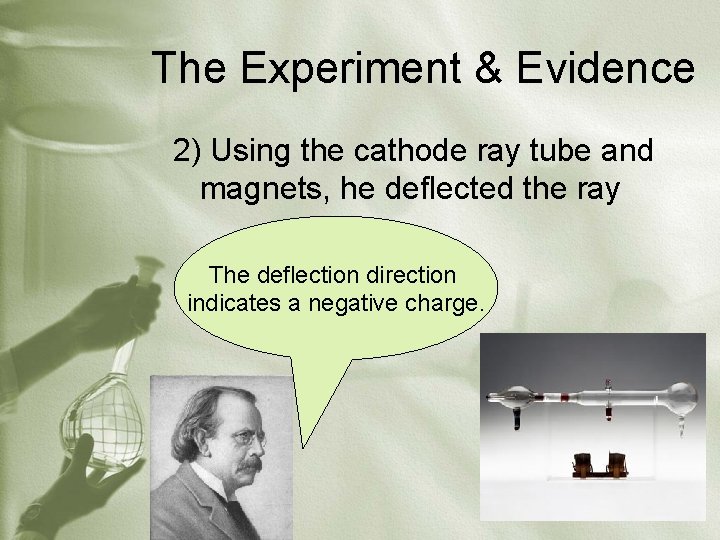
The Experiment & Evidence 2) Using the cathode ray tube and magnets, he deflected the ray The deflection direction indicates a negative charge.

The Experiment & Evidence 3) Thomson measures the mass-tocharge ratio of the rays by measuring how much it is deflected by the magnetic field & how much energy it carried The ratio is 1, 000 times smaller than that of the H+, these particles have less mass then the lightest atom known.

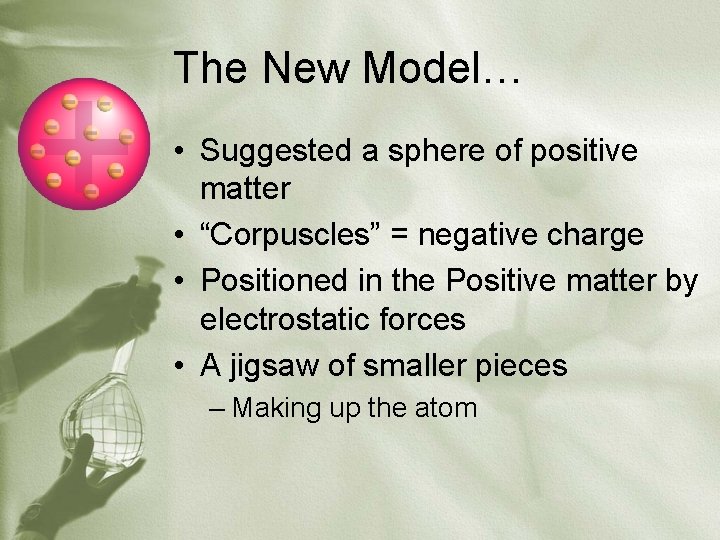
The New Model… • Suggested a sphere of positive matter • “Corpuscles” = negative charge • Positioned in the Positive matter by electrostatic forces • A jigsaw of smaller pieces – Making up the atom

Bibliography • • http: //www. chemheritage. org/classroom/chemach/atomic/thomson. html, retrieved June 15, 2009 http: //nobelprize. org/nobel_prizes/physics/laureates/1906/thomsonbio. html http: //www. atomicarchive. com/Bios/Thomson. shtml http: //en. wikipedia. org/wiki/J. _Thomson Images: • www. makingthemodernworld. org. uk/. . . /IC. 026/ • https: //reich-chemistry. wikispaces. com/file/view/348 px. Plum_pudding_atom_svg. png • http: //www. welsch. com/gallery/bitmap/Rosinenkuchen_nach__Thomps on_077. jpg • prl. aps. org/. . . /te 6? height=600%3 B&width=500%3 B • http: //nobelprize. org/nobel_prizes/physics/laureates/1906/thomsonbio. html • http: //www. chemheritage. org/Educational. Services/webquest/dalton. htm • www. aip. org/history/electron/jjcrooke. htm • www. manep. ch/. . . /nanotubes. html