Thermochemistry Feeling hot hot Thermochemistry concerned with the



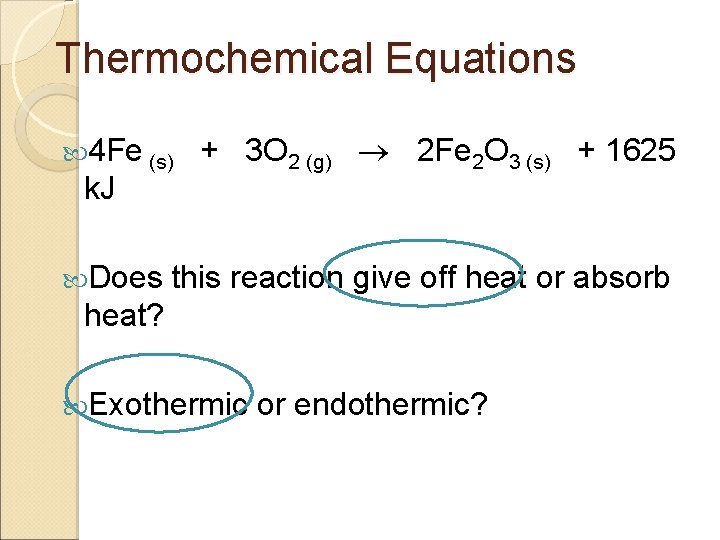





- Slides: 17

Thermochemistry Feeling hot, hot

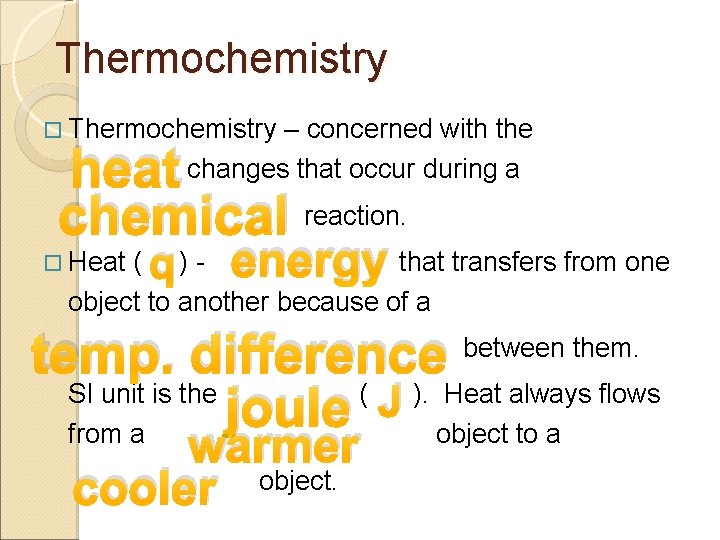
Thermochemistry – concerned with the changes that occur during a heat chemical reaction. Heat q energy ( )that transfers from one object to another because of a temp. difference between them. SI unit is the ( J ). Heat always flows joule from a object to a warmer cooler object.

Heat transfer Example: You place an ice cube into a bowl of hot soup. Describe the direction of heat flow. The hotter soup transfers heat to the colder ice. It is possible to transfer HEAT, but never COLD. (There is no such thing as cold transfer!)

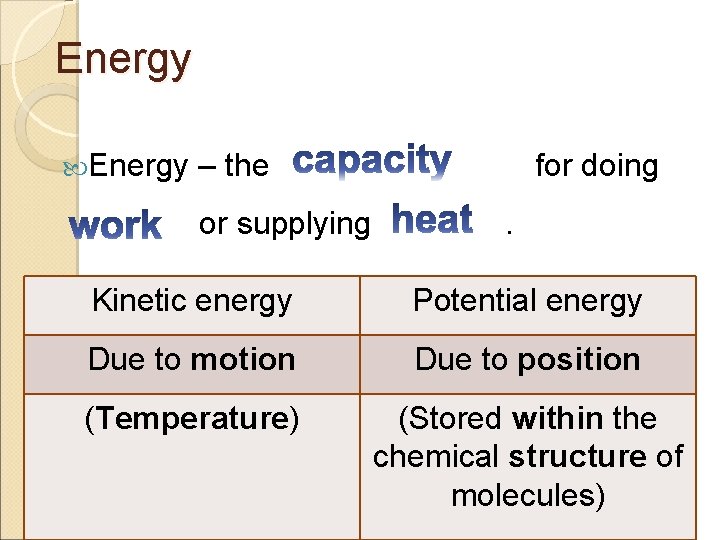
Energy – the or supplying for doing. Kinetic energy Potential energy Due to motion Due to position (Temperature) (Stored within the chemical structure of molecules)

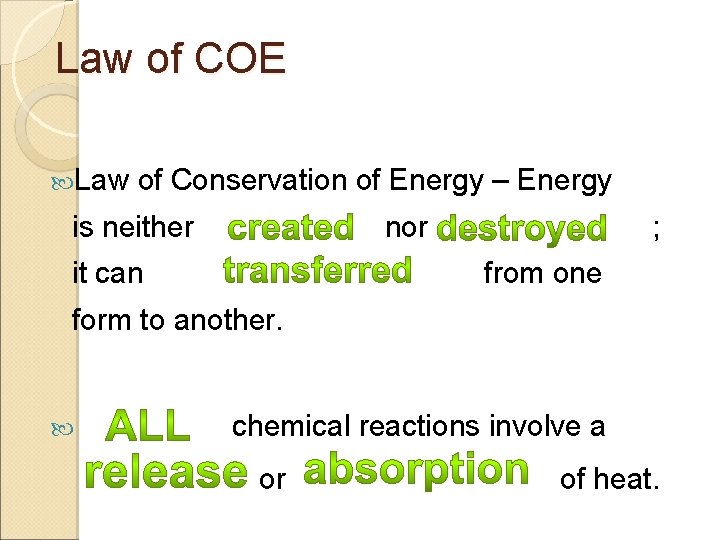
Law of COE Law of Conservation of Energy – Energy is neither it can nor. ; from one form to another. chemical reactions involve a or of heat.

Reactions exo - exit Exothermic process – releases heat to its surroundings Endothermic process – absorbs to its surroundings endo - enter heat

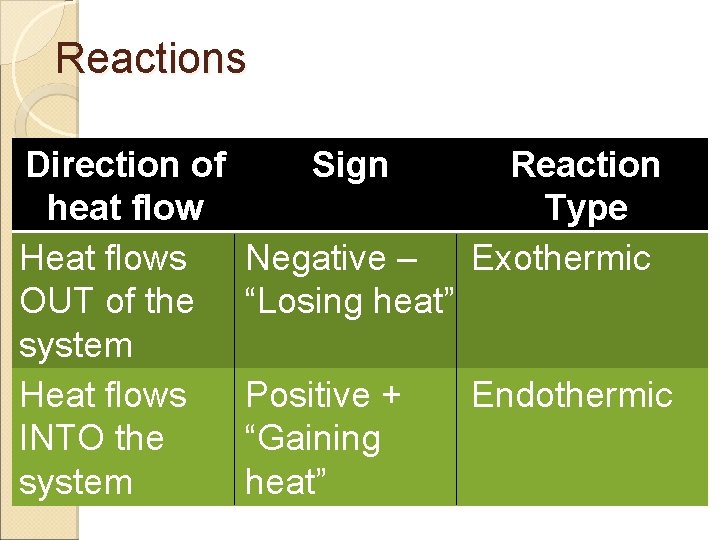
Reactions Direction of heat flow Heat flows OUT of the system Heat flows INTO the system Sign Negative – “Losing heat” Positive + “Gaining heat” Reaction Type Exothermic Endothermic
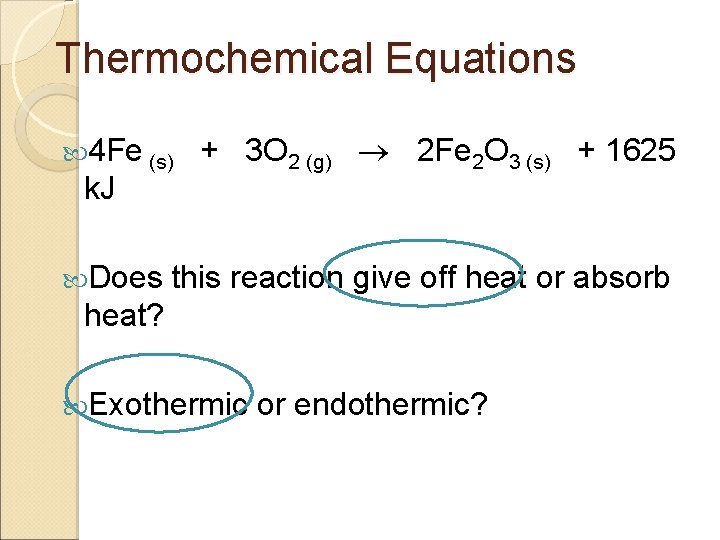
Thermochemical Equations 4 Fe (s) k. J Does + 3 O 2 (g) 2 Fe 2 O 3 (s) + 1625 this reaction give off heat or absorb heat? Exothermic or endothermic?

Thermochemical Equations What does k. J mean? Kilojoule = 1000 J (measurement heat of ) Enthalpy ΔH - change in heat content for a reaction at constant pressure.

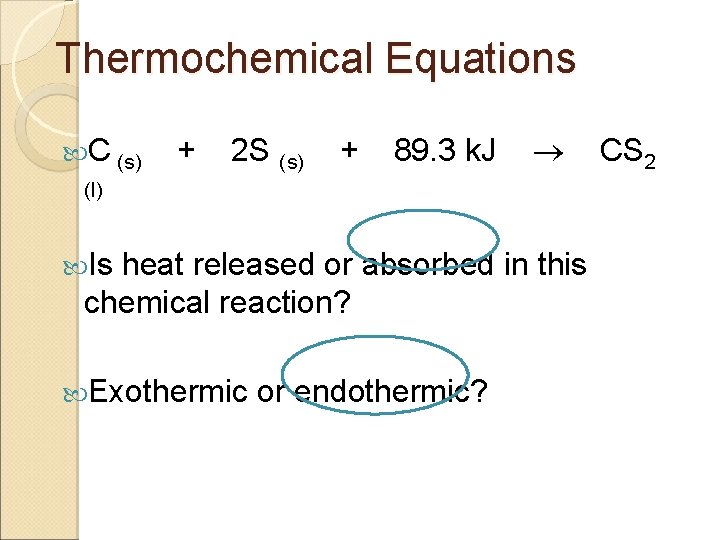
Thermochemical Equations C (s) + 2 S (s) + 89. 3 k. J (l) Is heat released or absorbed in this chemical reaction? Exothermic or endothermic? CS 2

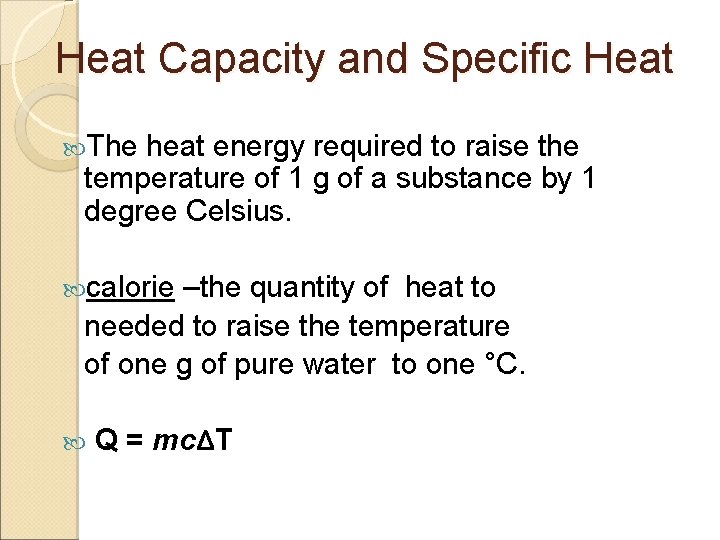
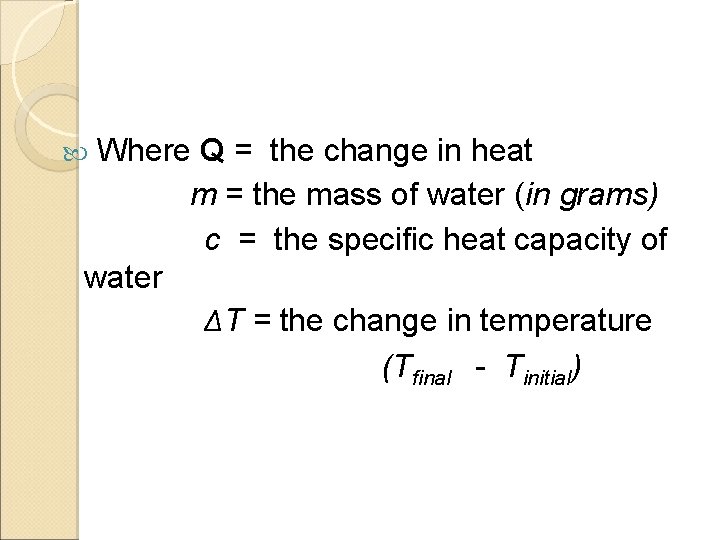
Heat Capacity and Specific Heat The heat energy required to raise the temperature of 1 g of a substance by 1 degree Celsius. calorie –the quantity of heat to needed to raise the temperature of one g of pure water to one °C. Q = mcΔT

Where Q = the change in heat m = the mass of water (in grams) c = the specific heat capacity of water ΔT = the change in temperature (T final - Tinitial)

Conversions Convert 444 calories to joules.

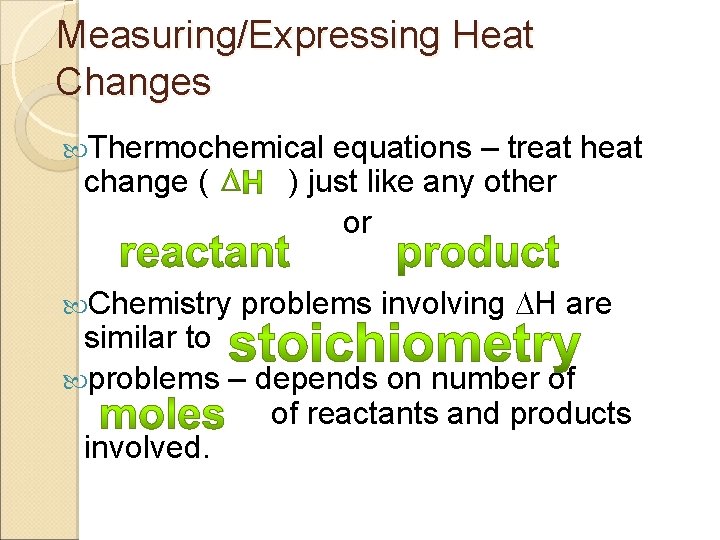
Measuring/Expressing Heat Changes Thermochemical change ( Chemistry equations – treat heat ) just like any other or problems involving H are similar to problems – depends on number of of reactants and products involved.

Energy Change in Exothermic and Endothermic Reaction

Calculate: Examples 1. Convert the following heat quantities recalling 1 cal= 4. 184 J a. 350 cal to joules b. 515 j to calories c. 1. 6 kcal to joules 2. How much heat is required to change the temperature of 150 g of water by 20 C? 3. How much heat will be given off when 1500 g of water cools down by 20 C?

You Do…. Pair What is the total number of joules of heat energy absorbed by 15 g of water when it is heated from 30 C to 40 C?
 Thermochemistry is concerned with the
Thermochemistry is concerned with the Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is the study of...
Thermochemistry is the study of... Hot nor hot
Hot nor hot White hot vs red hot temperature
White hot vs red hot temperature Cold working processes
Cold working processes Perbedaan hot lava dan hot lava volcano
Perbedaan hot lava dan hot lava volcano Công thức tiính động năng
Công thức tiính động năng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể độ dài liên kết
độ dài liên kết Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia điện thế nghỉ
điện thế nghỉ