Theories of the Atom What is a theory







- Slides: 15

Theories of the Atom

What is a theory? • A concise, predictive and broadly applicable explanation for a wide range of phenomena (Scientific observations) • It is not a “hunch” • They are constantly evolving

450 BCE: Earth, Water, Air and Fire - - - Idea supported by Greek philosopher Aristotle All matter is made of four basic substances: earth, water, air and fire The substances have specific qualities: dry, wet, cold and hot This was accepted for almost 2000 years

400 BCE: An Indivisible Particle Atom • Democritus develops the idea of atoms • suggested that matter was made up of tiny particles called: ATOMOS • Greek for indivisible

1807: Billiard Ball Model • John Dalton, English Scientist and teacher • working with gases, reconsidered Democritus’ theory that particles are indivisible • suggested that all matter was made up of tiny spheres that were able to bounce around with perfect elasticity and called them ATOMS

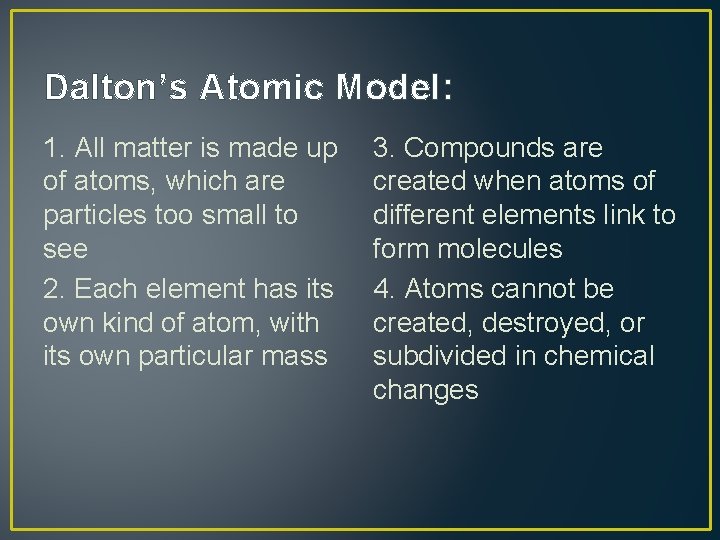
Dalton’s Atomic Model: 1. All matter is made up of atoms, which are particles too small to see 2. Each element has its own kind of atom, with its own particular mass 3. Compounds are created when atoms of different elements link to form molecules 4. Atoms cannot be created, destroyed, or subdivided in chemical changes

Problem with Dalton’s Theory: Unable to explain the Electrical nature of matter: Like charges repel Unlike charges attract

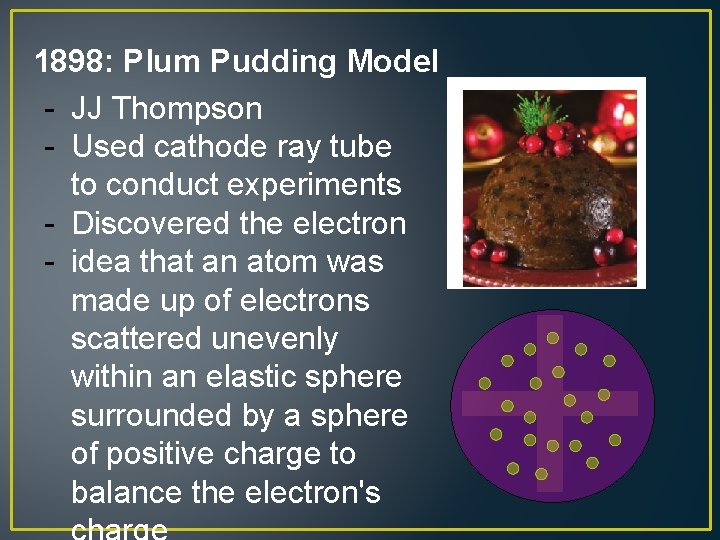
1898: Plum Pudding Model - JJ Thompson - Used cathode ray tube to conduct experiments - Discovered the electron - idea that an atom was made up of electrons scattered unevenly within an elastic sphere surrounded by a sphere of positive charge to balance the electron's

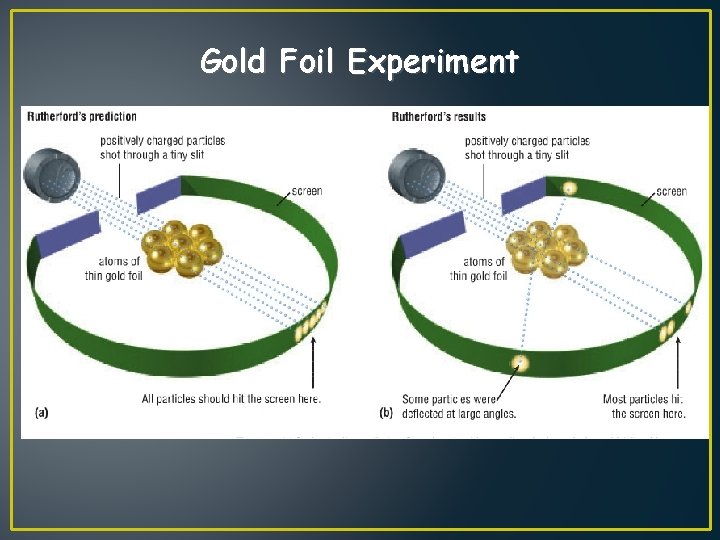
1910: Nucleus and Proton - Ernest Rutherford - designed an experiment using RADIUM (this element spits out positive ALPHA particles) - he placed a piece of gold foil in front of the beam, surrounded by a screen to detect the path of the particles - they found that although most of them passed through, about 1 in 10, 000 hit

Gold Foil Experiment helium nuclei gold foil helium nuclei

Rutherford’s Model - Centre of atom, called nucleus, has a positive charge - Nucleus occupies small space, but contains most of the atom’s mass - Cloud of negatively charged electrons surround the nucleus - Most of the atom is empty space - Credited for discovering the proton

1932: Planetary Model - James Chadwick, Rutherford’s student - Nucleus contains another particle which has NO charge (neutral) called a: NEUTRON - Atom is empty space with tiny, dense nucleus - Nucleus contains protons and neutrons - Electrons circle rapidly in the space around the nucleus - A neutral atom has the same number of protons and electrons

1913: Electron Orbits - refined Rutherford's idea by adding that the electrons were in orbits - Each orbit is only able to contain a set number of electrons.

Bohr-Rutherford Model • Explains properties of first 20 elements 1. Electrons orbit the nucleus 2. Each electron in an orbit has a definite amount of energy 3. The farther an electron is from the nucleus, the greater the energy 4. Electrons cannot be between orbits but they can jump to and from different orbits 5. Each orbit can hold a maximum number of electrons

Bohr-Rutherford Model
 The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Gambar atom democritus
Gambar atom democritus Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worms-breton
Tư thế worms-breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99