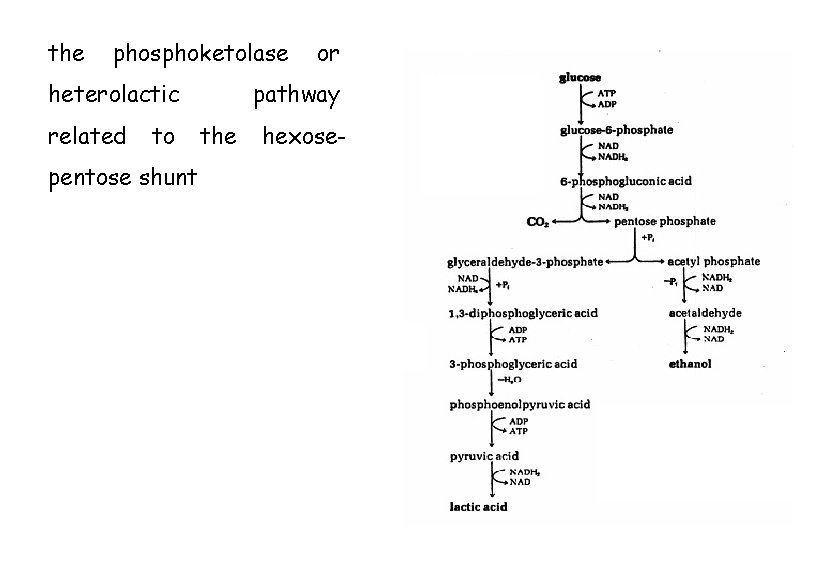
the phosphoketolase heterolactic related to pentose shunt or








- Slides: 24


the phosphoketolase heterolactic related to pentose shunt or pathway the hexose-

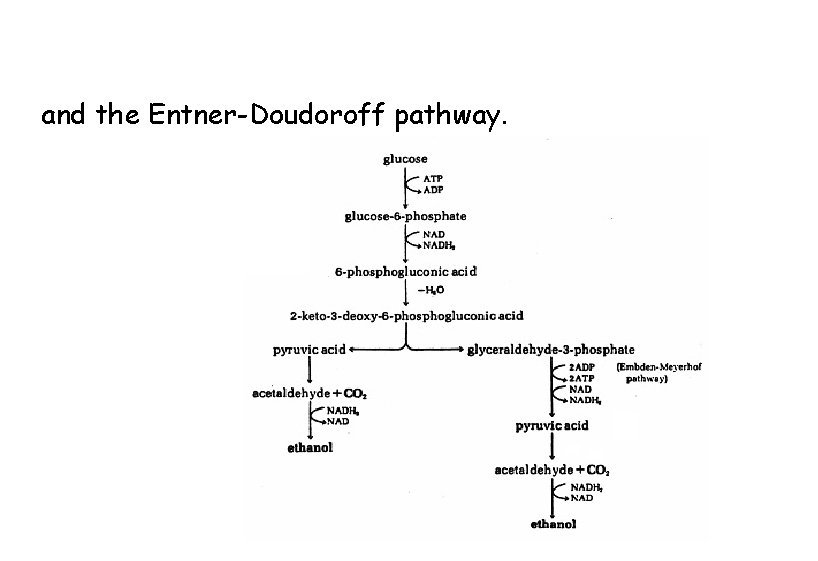
and the Entner-Doudoroff pathway.

For respiration to occur, in addition to the pathway of glycolysis, other essential metabolic components are needed: The tricarboxylic acid (TCA) cycle (also known as the citric acid cycle or the Kreb's cycle) when an organic compound is utilized as a substrate, the TCA cycle is required for the complete oxidation of the substrate. The end product that always results from the complete oxidation of an organic compound is CO 2.


A large amount of reduced NADH + H+ is generated by these reactions, and it must be reoxidized for metabolism to continue. In respiration, this occurs in membrane-associated electron transport systems.

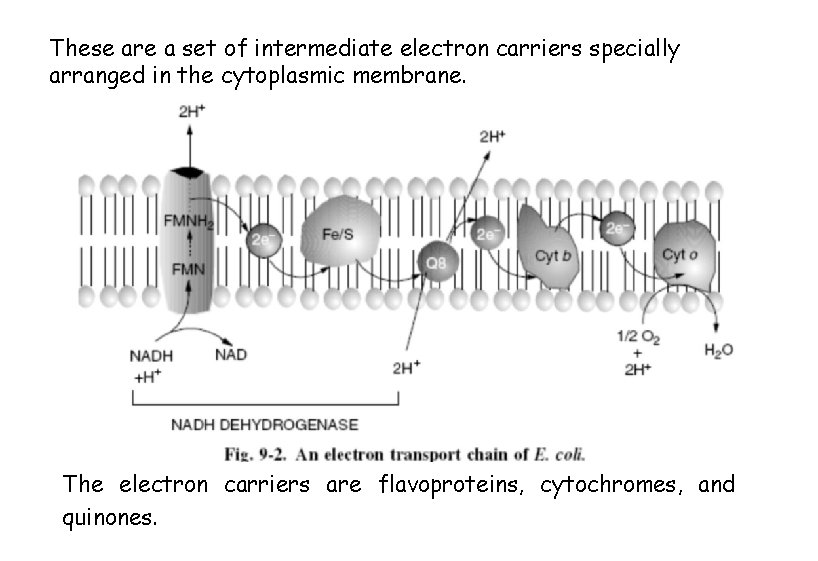
These are a set of intermediate electron carriers specially arranged in the cytoplasmic membrane. The electron carriers are flavoproteins, cytochromes, and quinones.

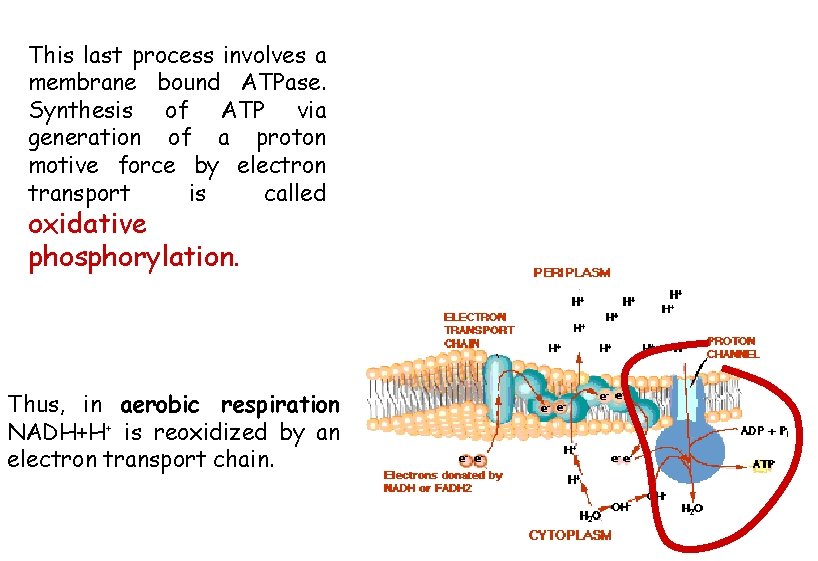
The specific arrangement of carriers in the membrane is such that as electrons and H+ are passed from carrier to carrier, the protons are extruded outside the cell membrane while the electrons are resumed to the cytoplasmic side. The result is a proton gradient, in which the outside face of the membrane has a lower p. H and a more positive charge. This energized state of the membrane (the proton motive force) represents potential energy that can perform useful work. The controlled passage of protons back across the membrane through specifíc membrane proteins is used to drive ion transport, flagellar rotation, or ATP synthesis.

This last process involves a membrane bound ATPase. Synthesis of ATP via generation of a proton motive force by electron transport is called oxidative phosphorylation. Thus, in aerobic respiration NADH+H+ is reoxidized by an electron transport chain.

3. An outside electron acceptor ("outside", meaning it is not internal to the pathway, as is pyruvate in a fermentation) is needed. For aerobic respiration the electron acceptor is O 2, of course. Molecular oxygen is reduced to H 20 in the last step of the electron transport system.

Respiration in some procaryotes is entirely possible using electron acceptors other than oxygen (O 2). This type of respiration in the absence of oxygen is referred to as anaerobic respiration.

Electron acceptors used by procaryotes for anaerobic respiration or methanogenesis (an analogous type of energy generation in archaea) Nitrate (NO 3 -) Sulfate (SO 42 -)

Nitrate (NO 3 -). Process called denitrification. Also called dissimilative nitrate reduction. Reduced waste products are excreted in significant amounts. Redox potential is + 0. 42 v (compared to + 0. 82 v for oxygen). So organisms respiring anaerobically gain less energy than with oxygen.


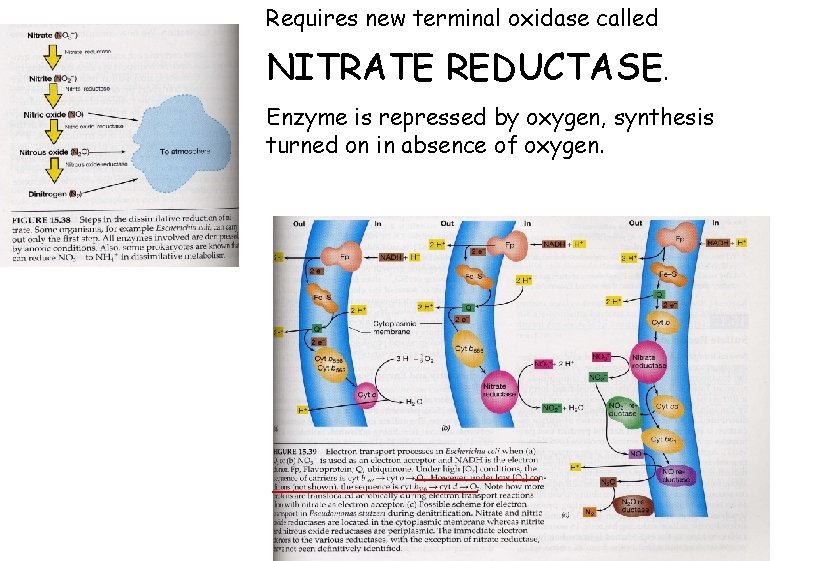
Requires new terminal oxidase called NITRATE REDUCTASE. Enzyme is repressed by oxygen, synthesis turned on in absence of oxygen.

Process can have several steps, proceed in two different directions: (A) nitrate (NO 3 -) ---> nitrite (NO 2 -) (E. coli) (B) nitrate (NO 3 -) ---> nitrite (NO 2 -) ---> nitrous oxide (N 2 O) ---> dinitrogen gas (N 2) (Pseudomonas) Second process is major pathway for loss of nitrogen compounds from soil, return of nitrogen to atmosphere. Pseudomonas species are common denitrifiers, widespread in soils. When fertilized soils become flooded, oxygen is rapidly depleted, pseudomonads switch to anaerobic respiration and can use up soil nitrate, leaving field in unfertile state.

Sulfate (SO 42 -). Process called sulfate reduction. Sulfate (SO 42 -). ---> Hydrogen Sulfide (H 2 S) Small group of bacteria carry out this reaction; all obligate anaerobes. Have unique cytochrome c 3. Sulfate is common in seawater. Often, H 2 S combines with iron, forms insoluble Fe. S ----> black sediments. Is common in estuaries.

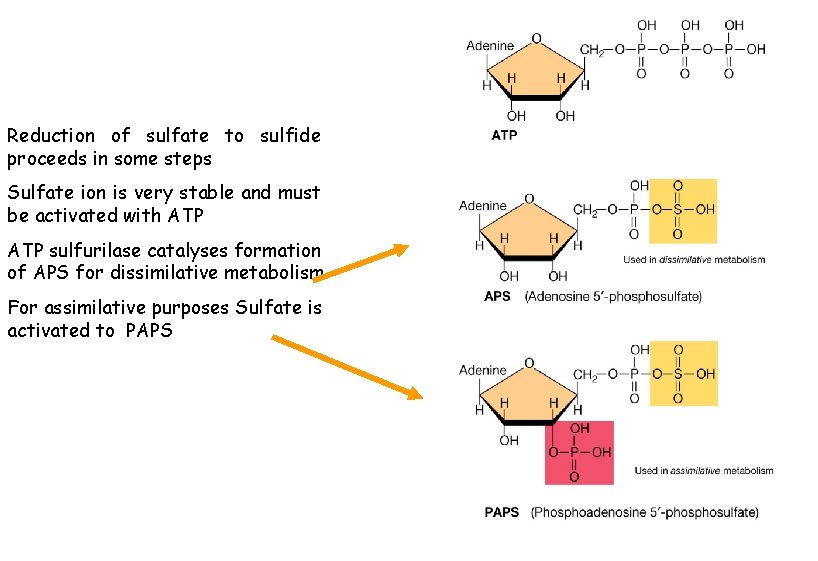
Reduction of sulfate to sulfide proceeds in some steps Sulfate ion is very stable and must be activated with ATP sulfurilase catalyses formation of APS for dissimilative metabolism For assimilative purposes Sulfate is activated to PAPS


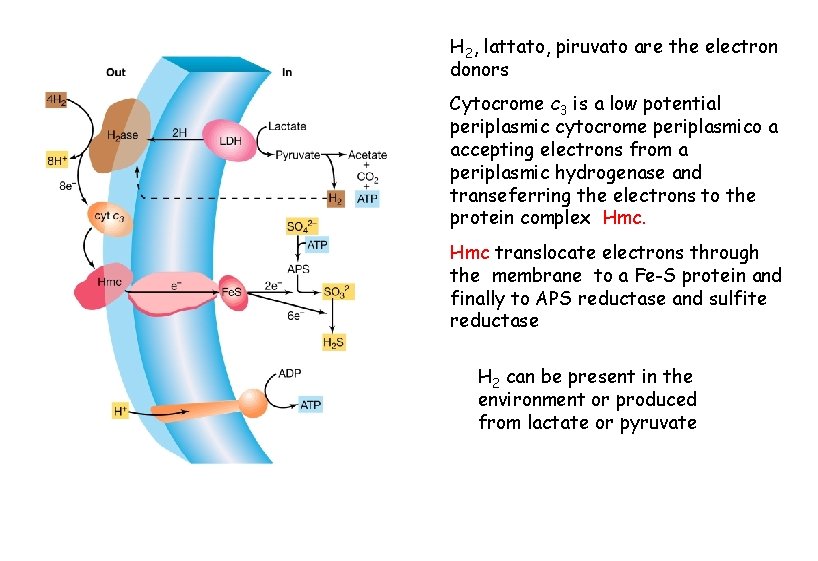
H 2, lattato, piruvato are the electron donors Cytocrome c 3 is a low potential periplasmic cytocrome periplasmico a accepting electrons from a periplasmic hydrogenase and transeferring the electrons to the protein complex Hmc translocate electrons through the membrane to a Fe-S protein and finally to APS reductase and sulfite reductase H 2 can be present in the environment or produced from lactate or pyruvate

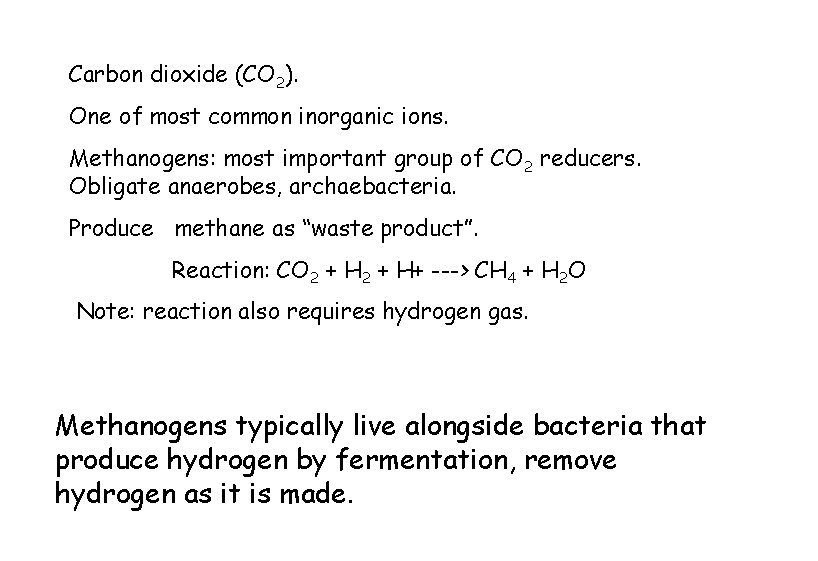
Carbon dioxide (CO 2). One of most common inorganic ions. Methanogens: most important group of CO 2 reducers. Obligate anaerobes, archaebacteria. Produce methane as “waste product”. Reaction: CO 2 + H+ ---> CH 4 + H 2 O Note: reaction also requires hydrogen gas. Methanogens typically live alongside bacteria that produce hydrogen by fermentation, remove hydrogen as it is made.



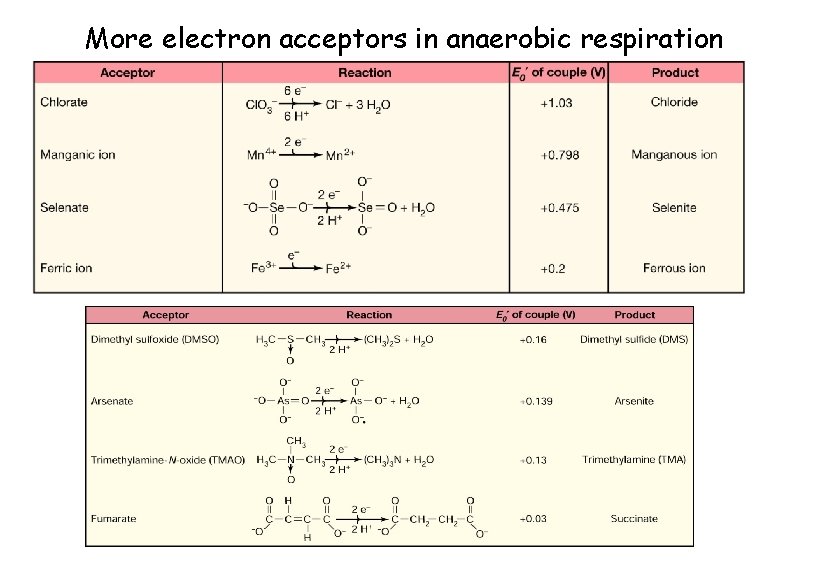
More electron acceptors in anaerobic respiration
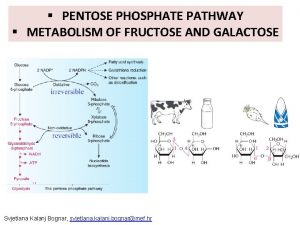
 Importance of pentose phosphate pathway
Importance of pentose phosphate pathway Plant glycolysis
Plant glycolysis Heterolactic fermentation pathway
Heterolactic fermentation pathway Phosphoketolase pathway
Phosphoketolase pathway Phosphoketolase pathway
Phosphoketolase pathway Shunt series feedback system diagram
Shunt series feedback system diagram Pentose phosphate pathway
Pentose phosphate pathway Non-oxidative phase of pentose phosphate pathway
Non-oxidative phase of pentose phosphate pathway Pentose sugar structure in dna
Pentose sugar structure in dna Pentose phosphate pathway
Pentose phosphate pathway Gluconeogenesis meaning
Gluconeogenesis meaning Monosaccharide
Monosaccharide Amidophosphoribosyltransferase
Amidophosphoribosyltransferase Barfoed's test reagent
Barfoed's test reagent Difference between hexose and pentose
Difference between hexose and pentose Skill and health related
Skill and health related What are the two types of physical components
What are the two types of physical components Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập đại từ thay thế
đại từ thay thế Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Phép trừ bù
Phép trừ bù Công thức tính thế năng
Công thức tính thế năng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi