The overwhelming case for LDLC lowering Prof Kausik






- Slides: 27

The overwhelming case for LDL-C lowering Prof Kausik Ray, BSc (hons), MBCh. B, FRCP, MD, MPhil (Cantab), FACC, FESC Professor of Cardiovascular Disease Prevention St Georges University of London Honorary Consultant Cardiologist St Georges Hospital

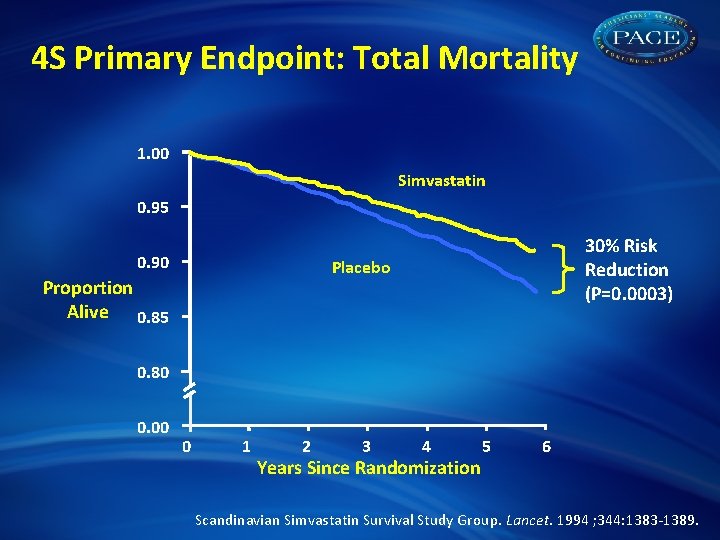
4 S Primary Endpoint: Total Mortality 1. 00 Simvastatin 0. 95 0. 90 30% Risk Reduction (P=0. 0003) Placebo Proportion Alive 0. 85 0. 80 0. 00 0 1 2 3 4 Years Since Randomization 5 6 Scandinavian Simvastatin Survival Study Group. Lancet. 1994 ; 344: 1383 -1389.

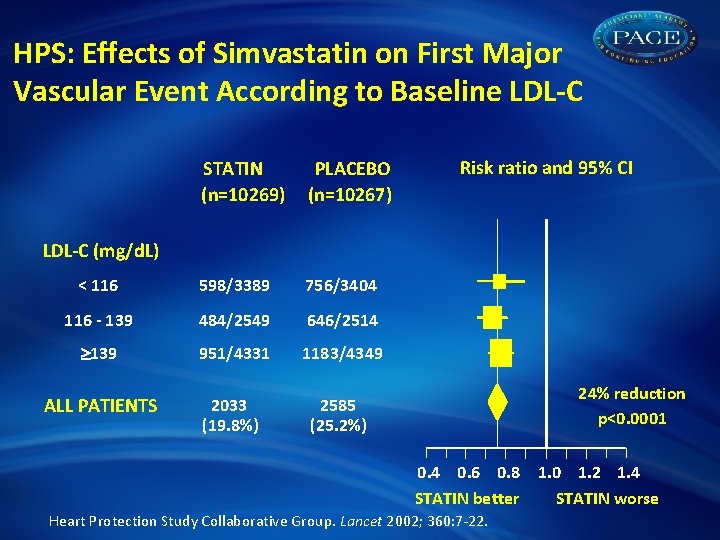
HPS: Effects of Simvastatin on First Major Vascular Event According to Baseline LDL-C STATIN (n=10269) PLACEBO (n=10267) < 116 598/3389 756/3404 116 - 139 484/2549 646/2514 139 951/4331 1183/4349 ALL PATIENTS 2033 (19. 8%) 2585 (25. 2%) Risk ratio and 95% CI LDL-C (mg/d. L) 24% reduction p<0. 0001 0. 4 0. 6 0. 8 1. 0 1. 2 1. 4 STATIN better STATIN worse Heart Protection Study Collaborative Group. Lancet 2002; 360: 7 -22.

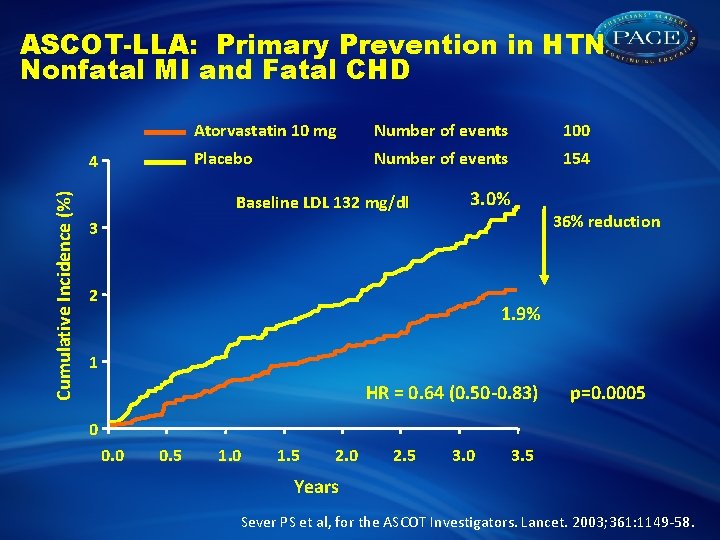
ASCOT-LLA: Primary Prevention in HTN Nonfatal MI and Fatal CHD Cumulative Incidence (%) 4 Atorvastatin 10 mg Number of events 100 Placebo Number of events 154 Baseline LDL 132 mg/dl 3. 0% 36% reduction 3 2 1. 9% 1 HR = 0. 64 (0. 50 -0. 83) p=0. 0005 0 0. 5 1. 0 1. 5 2. 0 2. 5 3. 0 3. 5 Years Sever PS et al, for the ASCOT Investigators. Lancet. 2003; 361: 1149 -58.

CARDS-Cumulative Hazard for Primary Endpoint Cumulative Hazard (%) 15 Relative Risk -37% (95% CI: -52, -17) P=0. 001 Placebo 127 events 10 Atorvastatin 10 mg 83 events 5 0 0 Placebo 1410 Atorva 1428 1 1351 1392 2 1306 1361 3 1022 1074 4 651 694 4. 75 305 328 Years

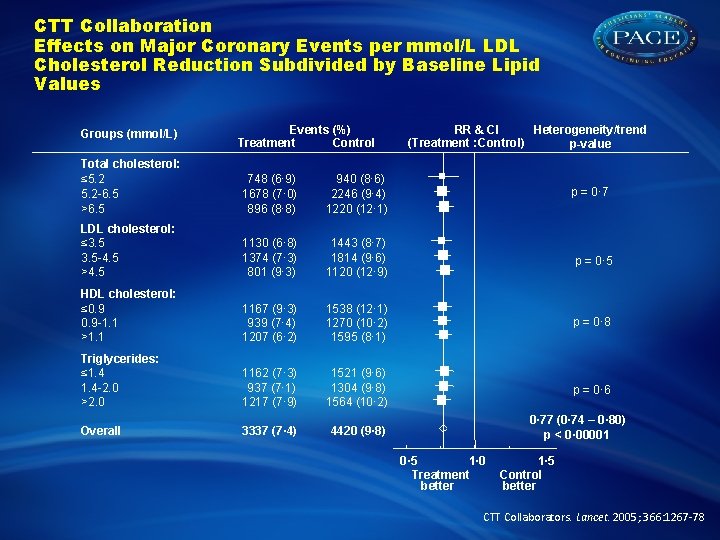
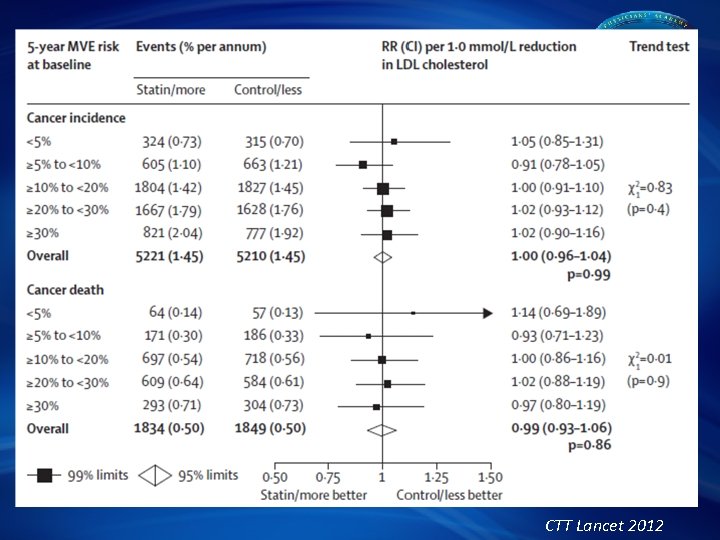
CTT Collaboration Effects on Major Coronary Events per mmol/L LDL Cholesterol Reduction Subdivided by Baseline Lipid Values Groups (mmol/L) Events (%) Treatment Control Heterogeneity/trend RR & CI (Treatment : Control) p-value Total cholesterol: ≤ 5. 2 -6. 5 >6. 5 748 (6· 9) 1678 (7· 0) 896 (8· 8) 940 (8· 6) 2246 (9· 4) 1220 (12· 1) LDL cholesterol: ≤ 3. 5 -4. 5 >4. 5 1130 (6· 8) 1374 (7· 3) 801 (9· 3) 1443 (8· 7) 1814 (9· 6) 1120 (12· 9) p = 0· 5 HDL cholesterol: ≤ 0. 9 -1. 1 >1. 1 1167 (9· 3) 939 (7· 4) 1207 (6· 2) 1538 (12· 1) 1270 (10· 2) 1595 (8· 1) p = 0· 8 Triglycerides: ≤ 1. 4 -2. 0 >2. 0 1162 (7· 3) 937 (7· 1) 1217 (7· 9) 1521 (9· 6) 1304 (9· 8) 1564 (10· 2) p = 0· 6 Overall 3337 (7· 4) 4420 (9· 8) p = 0· 77 (0· 74 – 0· 80) p < 0· 00001 0· 5 1· 0 Treatment better 1· 5 Control better CTT Collaborators. Lancet. 2005; 366: 1267 -78

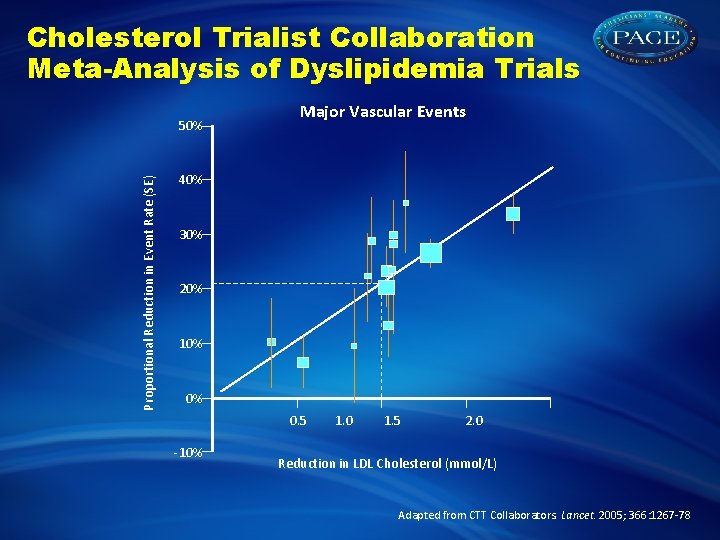
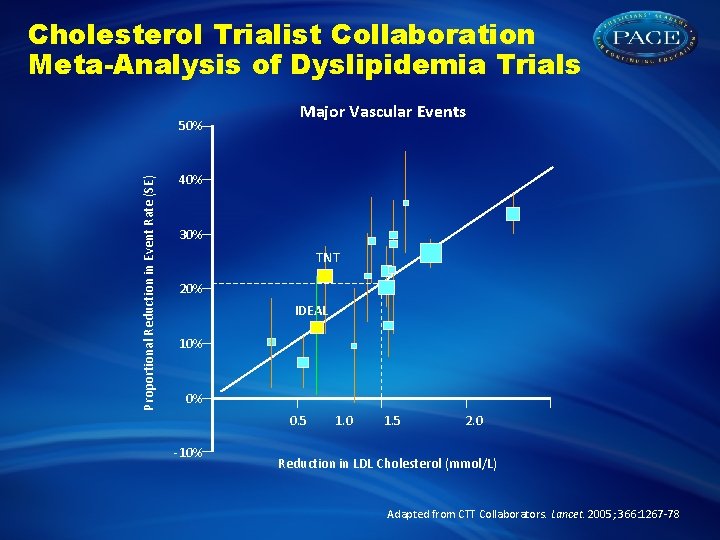
Cholesterol Trialist Collaboration Meta-Analysis of Dyslipidemia Trials Proportional Reduction in Event Rate (SE) 50% Major Vascular Events 40% 30% 20% 10% 0% 0. 5 -10% 1. 0 1. 5 2. 0 Reduction in LDL Cholesterol (mmol/L) Adapted from CTT Collaborators. Lancet. 2005; 366: 1267 -78

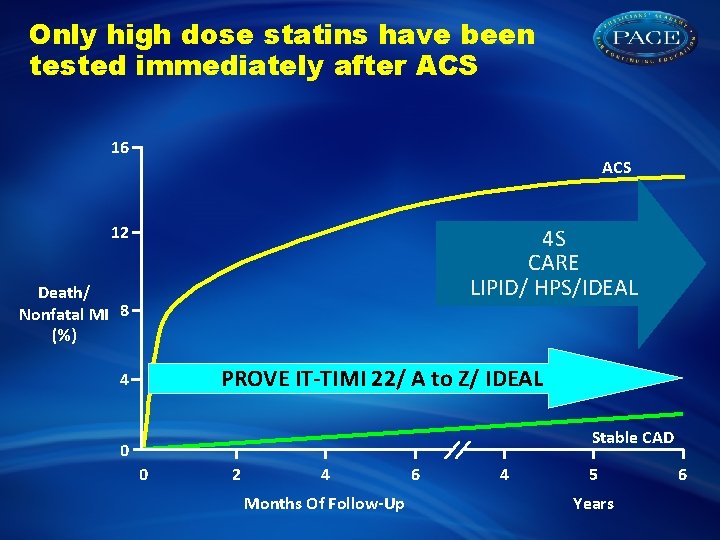
Only high dose statins have been tested immediately after ACS 16 ACS 12 4 S CARE LIPID/ HPS/IDEAL Death/ Nonfatal MI 8 (%) PROVE IT-TIMI 22/ A to Z/ IDEAL 4 Stable CAD 0 0 2 4 Months Of Follow-Up 6 4 5 Years 6

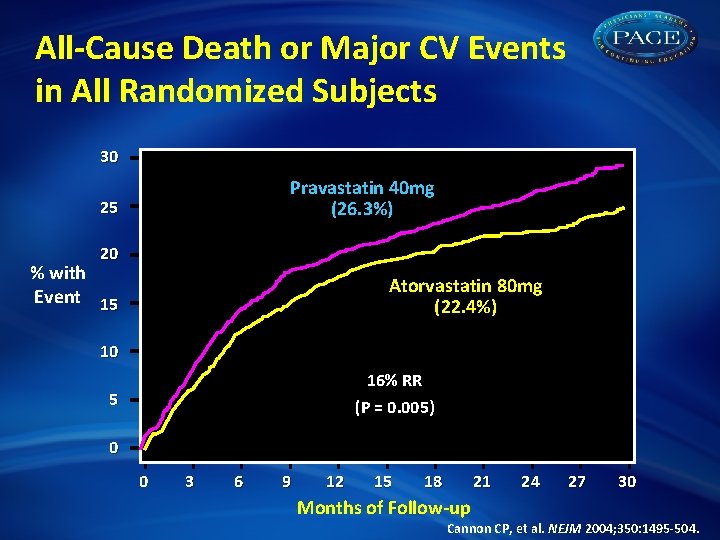
All-Cause Death or Major CV Events in All Randomized Subjects 30 Pravastatin 40 mg (26. 3%) 25 20 % with Event 15 Atorvastatin 80 mg (22. 4%) 10 16% RR (P = 0. 005) 5 0 0 3 6 9 12 15 18 21 24 27 30 Months of Follow-up Cannon CP, et al. NEJM 2004; 350: 1495 -504.

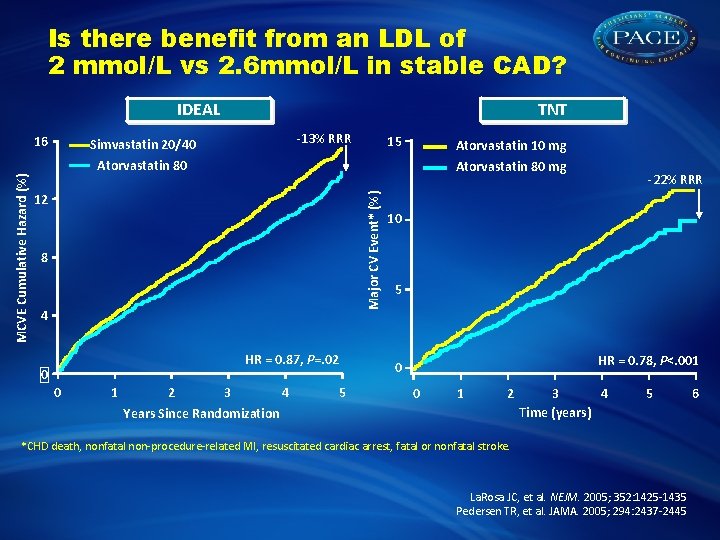
Is there benefit from an LDL of 2 mmol/L vs 2. 6 mmol/L in stable CAD? IDEAL -13% RRR Simvastatin 20/40 15 Atorvastatin 10 mg Atorvastatin 80 mg 12 Major CV Event* (%) MCVE Cumulative Hazard (%) 16 TNT 8 4 HR = 0. 87, P=. 02 0 0 1 2 3 4 Years Since Randomization 5 -22% RRR 10 5 HR = 0. 78, P<. 001 0 0 1 2 3 4 Time (years) 5 *CHD death, nonfatal non-procedure-related MI, resuscitated cardiac arrest, fatal or nonfatal stroke. La. Rosa JC, et al. NEJM. 2005; 352: 1425 -1435 Pedersen TR, et al. JAMA. 2005; 294: 2437 -2445 6

Cholesterol Trialist Collaboration Meta-Analysis of Dyslipidemia Trials Proportional Reduction in Event Rate (SE) 50% Major Vascular Events 40% 30% TNT 20% IDEAL 10% 0% 0. 5 -10% 1. 0 1. 5 2. 0 Reduction in LDL Cholesterol (mmol/L) Adapted from CTT Collaborators. Lancet. 2005; 366: 1267 -78

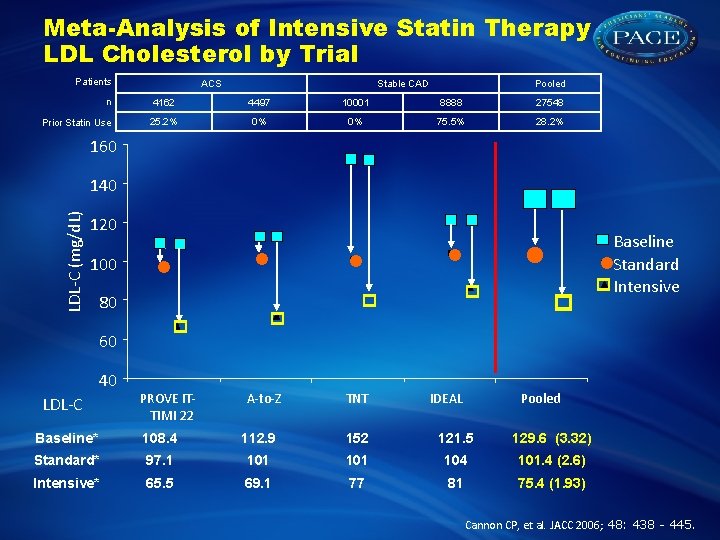
Meta-Analysis of Intensive Statin Therapy LDL Cholesterol by Trial Patients n Prior Statin Use ACS Stable CAD Pooled 4162 4497 10001 8888 27548 25. 2% 0% 0% 75. 5% 28. 2% 160 . LDL-C (mg/d. L) 140 120 100 80 60 40 LDL-C PROVE ITTIMI 22 A-to-Z TNT IDEAL Baseline Standard Intensive Pooled Baseline* 108. 4 112. 9 152 121. 5 129. 6 (3. 32) Standard* 97. 1 101 104 101. 4 (2. 6) Intensive* 65. 5 69. 1 77 81 75. 4 (1. 93) Cannon CP, et al. JACC 2006; 48: 438 - 445.

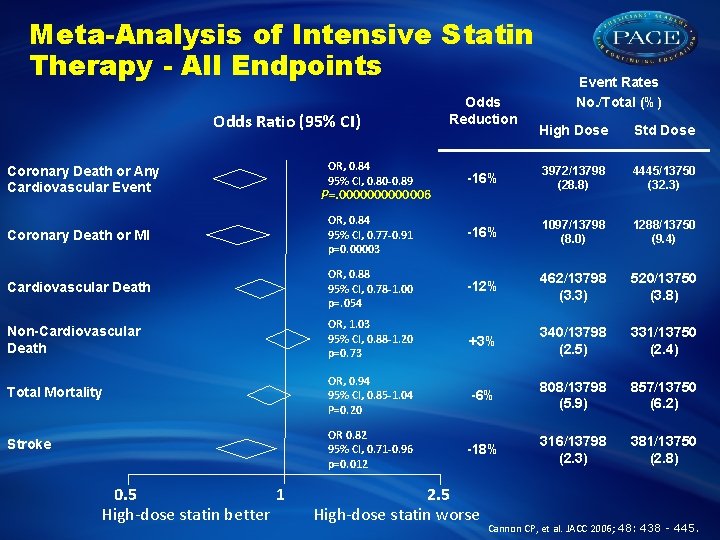
Meta-Analysis of Intensive Statin Therapy - All Endpoints Odds Ratio (95% CI) Odds Reduction Event Rates No. /Total (%) High Dose Std Dose OR, 0. 84 95% CI, 0. 80 -0. 89 -16% 3972/13798 (28. 8) 4445/13750 (32. 3) Coronary Death or MI OR, 0. 84 95% CI, 0. 77 -0. 91 p=0. 00003 -16% 1097/13798 (8. 0) 1288/13750 (9. 4) Cardiovascular Death OR, 0. 88 95% CI, 0. 78 -1. 00 p=. 054 -12% 462/13798 (3. 3) 520/13750 (3. 8) Non-Cardiovascular Death OR, 1. 03 95% CI, 0. 88 -1. 20 p=0. 73 +3% 340/13798 (2. 5) 331/13750 (2. 4) Total Mortality OR, 0. 94 95% CI, 0. 85 -1. 04 P=0. 20 -6% 808/13798 (5. 9) 857/13750 (6. 2) Stroke OR 0. 82 95% CI, 0. 71 -0. 96 p=0. 012 -18% 316/13798 (2. 3) 381/13750 (2. 8) Coronary Death or Any Cardiovascular Event 0. 5 1 High-dose statin better P=. 0000006 2. 5 High-dose statin worse Cannon CP, et al. JACC 2006; 48: 438 - 445.

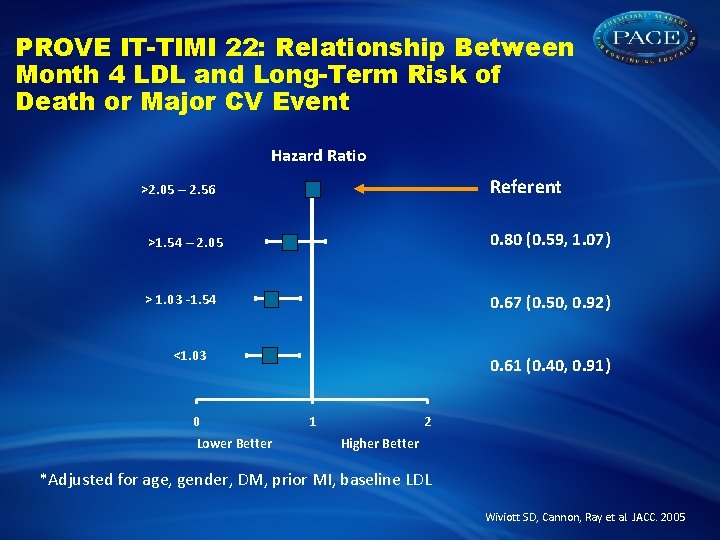
PROVE IT-TIMI 22: Relationship Between Month 4 LDL and Long-Term Risk of Death or Major CV Event Hazard Ratio Referent >2. 05 – 2. 56 >1. 54 – 2. 05 0. 80 (0. 59, 1. 07) > 1. 03 -1. 54 0. 67 (0. 50, 0. 92) <1. 03 0 Lower Better 0. 61 (0. 40, 0. 91) 1 2 Higher Better *Adjusted for age, gender, DM, prior MI, baseline LDL Wiviott SD, Cannon, Ray et al. JACC. 2005

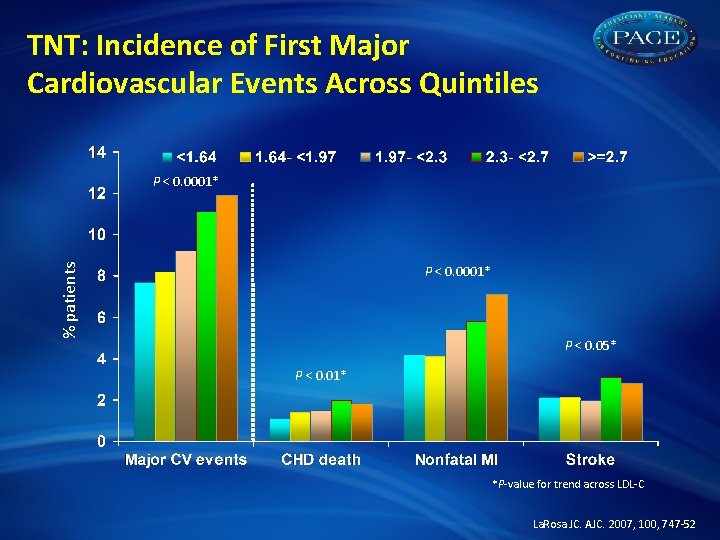
TNT: Incidence of First Major Cardiovascular Events Across Quintiles % patients P < 0. 0001* P < 0. 05* P < 0. 01* *P-value for trend across LDL-C La. Rosa JC. AJC. 2007, 100, 747 -52




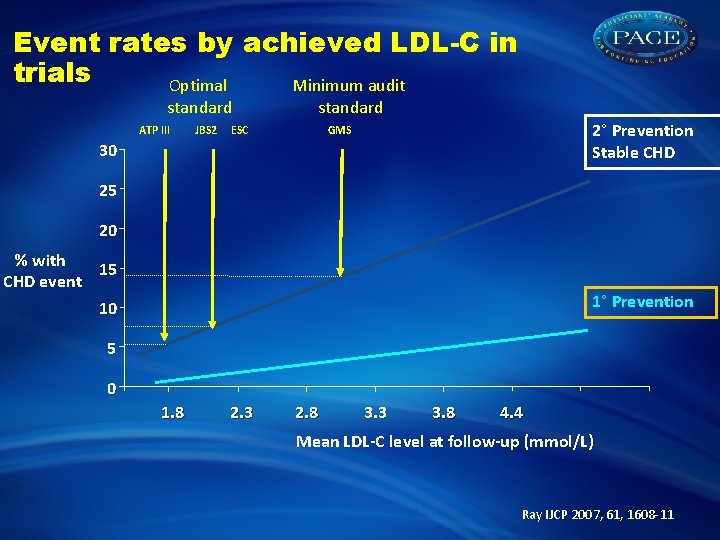
Event rates by achieved LDL-C in trials Optimal Minimum audit standard ATP III JBS 2 standard ESC 2° Prevention Stable CHD GMS 30 25 20 % with 15 CHD event 1° Prevention 10 5 0 1. 8 2. 3 2. 8 3. 3 3. 8 4. 4 Mean LDL-C level at follow-up (mmol/L) Ray IJCP 2007, 61, 1608 -11

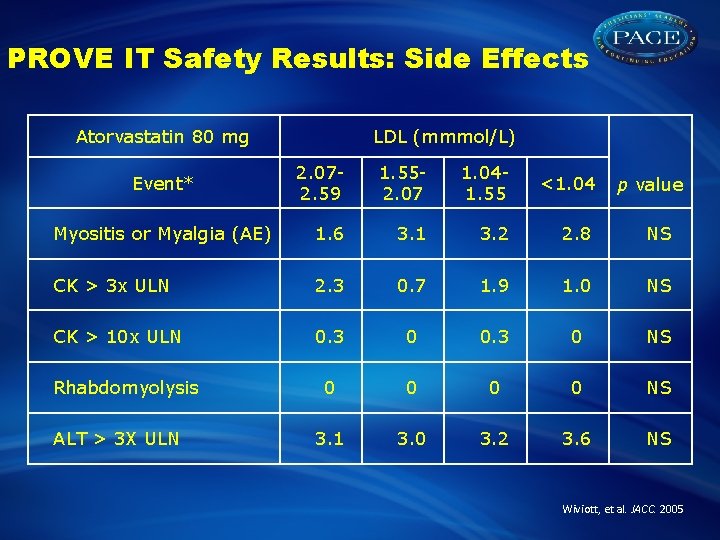
PROVE IT Safety Results: Side Effects Atorvastatin 80 mg LDL (mmmol/L) 2. 072. 59 1. 552. 07 1. 041. 55 <1. 04 Myositis or Myalgia (AE) 1. 6 3. 1 3. 2 2. 8 NS CK > 3 x ULN 2. 3 0. 7 1. 9 1. 0 NS CK > 10 x ULN 0. 3 0 NS 0 0 NS 3. 1 3. 0 3. 2 3. 6 NS Event* Rhabdomyolysis ALT > 3 X ULN p value Wiviott, et al. JACC. 2005

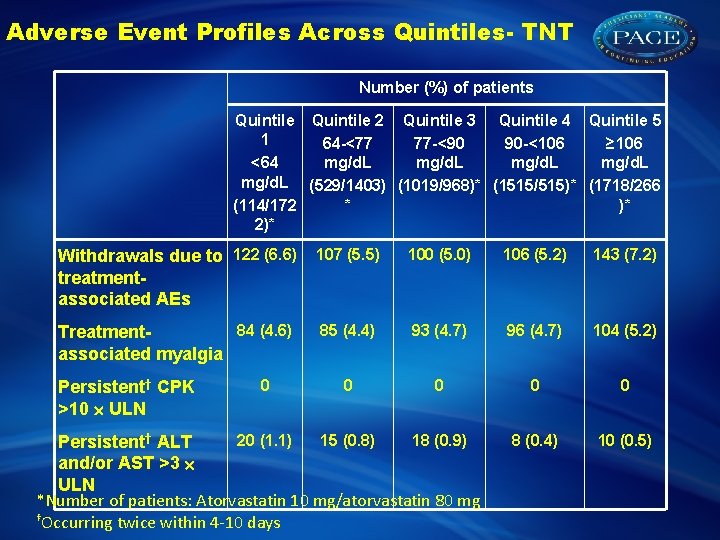
Adverse Event Profiles Across Quintiles- TNT Number (%) of patients Quintile 2 Quintile 3 Quintile 4 Quintile 5 1 64 -<77 77 -<90 90 -<106 ≥ 106 mg/d. L <64 mg/d. L (529/1403) (1019/968)* (1515/515)* (1718/266 * )* (114/172 2)* Withdrawals due to 122 (6. 6) treatmentassociated AEs 107 (5. 5) 100 (5. 0) 106 (5. 2) 143 (7. 2) 84 (4. 6) Treatmentassociated myalgia 85 (4. 4) 93 (4. 7) 96 (4. 7) 104 (5. 2) 0 0 8 (0. 4) 10 (0. 5) Persistent† CPK >10 ULN 0 20 (1. 1) 15 (0. 8) 18 (0. 9) Persistent† ALT and/or AST >3 ULN *Number of patients: Atorvastatin 10 mg/atorvastatin 80 mg †Occurring twice within 4 -10 days

CTT Lancet 2012

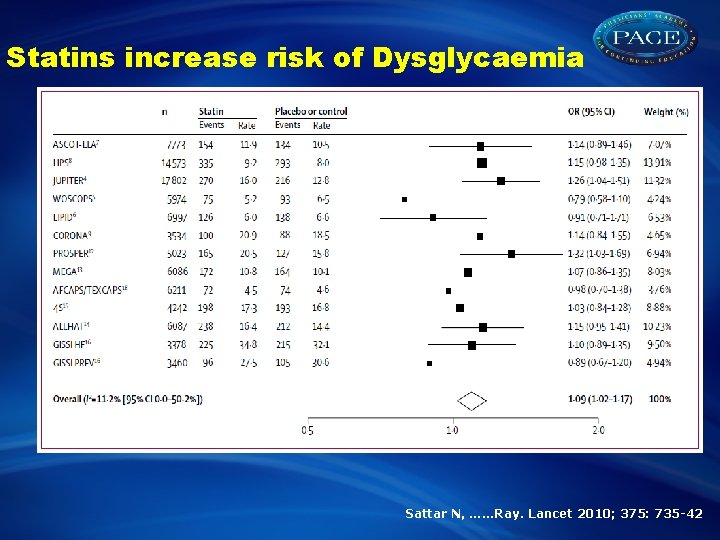
Statins increase risk of Dysglycaemia Sattar N, ……Ray. Lancet 2010; 375: 735 -42

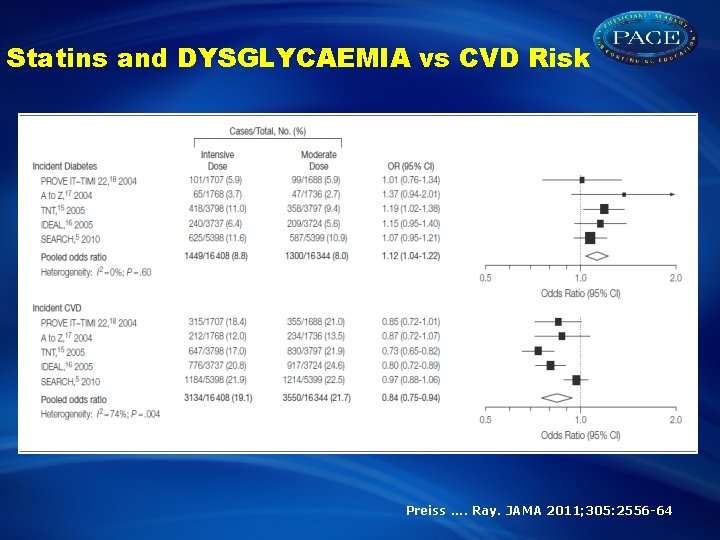
Statins and DYSGLYCAEMIA vs CVD Risk Preiss. . Ray. JAMA 2011; 305: 2556 -64

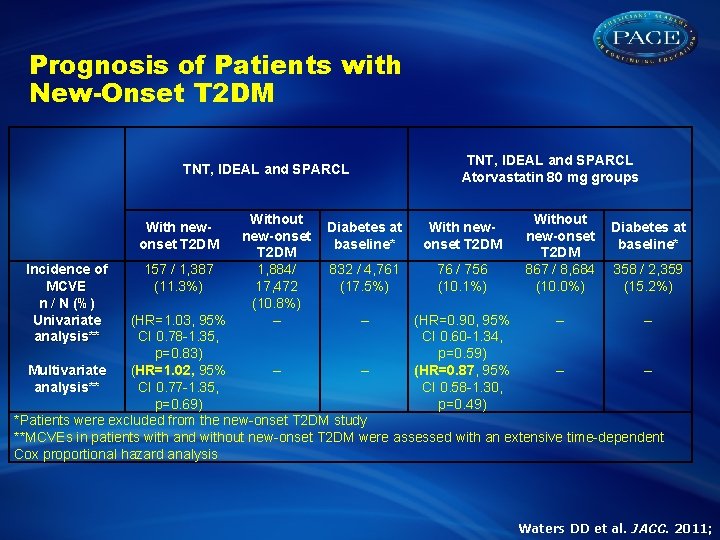
Prognosis of Patients with New-Onset T 2 DM TNT, IDEAL and SPARCL With newonset T 2 DM Incidence of MCVE n / N (%) Univariate analysis** 157 / 1, 387 (11. 3%) Without new-onset T 2 DM 1, 884/ 17, 472 (10. 8%) – TNT, IDEAL and SPARCL Atorvastatin 80 mg groups Diabetes at baseline* With newonset T 2 DM 832 / 4, 761 (17. 5%) 76 / 756 (10. 1%) Without new-onset T 2 DM 867 / 8, 684 (10. 0%) Diabetes at baseline* 358 / 2, 359 (15. 2%) (HR=1. 03, 95% – (HR=0. 90, 95% – – CI 0. 78 -1. 35, CI 0. 60 -1. 34, p=0. 83) p=0. 59) Multivariate (HR=1. 02, 95% – – (HR=0. 87, 95% – – analysis** CI 0. 77 -1. 35, CI 0. 58 -1. 30, p=0. 69) p=0. 49) *Patients were excluded from the new-onset T 2 DM study **MCVEs in patients with and without new-onset T 2 DM were assessed with an extensive time-dependent Cox proportional hazard analysis Waters DD et al. JACC. 2011;

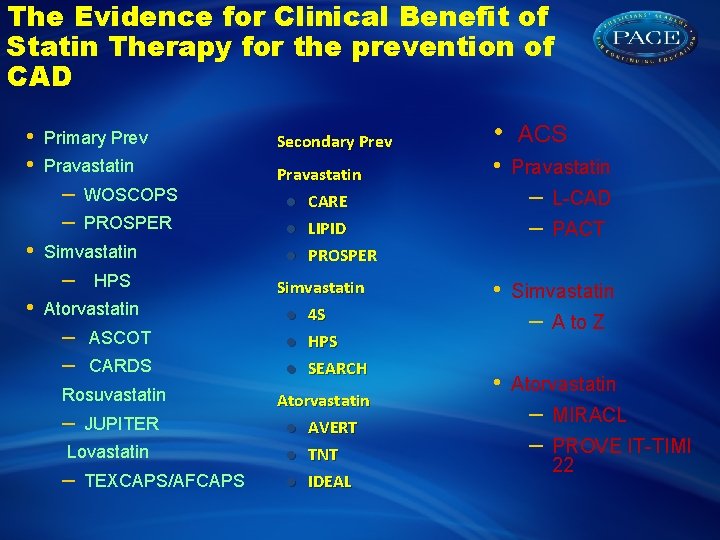
The Evidence for Clinical Benefit of Statin Therapy for the prevention of CAD • • Primary Prev Secondary Prev Pravastatin CARE LIPID PROSPER – – • PROSPER Simvastatin – • WOSCOPS HPS Atorvastatin – – ASCOT CARDS Rosuvastatin – JUPITER Lovastatin – TEXCAPS/AFCAPS Simvastatin 4 S HPS SEARCH Atorvastatin AVERT TNT IDEAL • • ACS Pravastatin – – • PACT Simvastatin – • L-CAD A to Z Atorvastatin – – MIRACL PROVE IT-TIMI 22

Summary • LDL-C lowering offers similar proportional reductions among those with and without vascular disease • The magnitude of benefit is related to the degree of LDL-C reduction • LDL-C lowering is safe in general • The greatest benefit are in those with the greatest absolute risk