The clinical relevance of luteal phase deficiency a


































- Slides: 44

The clinical relevance of luteal phase deficiency: a committee opinion Fertility and Sterility Vol. 98, No. 5, November 2012 Presenter: R 4孫怡虹 Advisor: VS鍾明廷

INTRODUCTION

Maintenance of pregnancy • Corpus luteum Progesterone – After ovulation ~ during the early first trimester ~ until placental function established – Removal of the corpus luteum spontaneous pregnancy loss • Ovarian progesterone production implantation & early pregnancy

- Ovarian inadequacy • As a cause of infertility or pregnancy failure ? Cycles (Conception occurs vs. no conception): • More rapid rise of progesterone • ↑ mid-luteal estrogen and progesterone levels Cycles (Normal vs. biochemical pregnancies): • Similarly luteal phase progesterone levels • Pregnancy losses do not always result from ovarian insufficiency

- Delayed implantation • Associated with ↑Pregnancy loss • More likely a result of an embryonic problem with inadequate early h. CG production • Rather than an inappropriate ovarian response

Luteal phase deficiency (LPD) • Endogenous progesterone is not sufficient to – Maintain a functional secretory endometrium – Allow normal embryo implantation and growth – 1 st described in 1949

Luteal Phase Deficiency (LPD) Purportedly been associated with: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Infertility 1 st trimester pregnancy loss Short cycles Premenstrual spotting Anorexia Starvation Eating disorders Excessive exercise Stress Obesity & PCOS 11. Endometriosis 12. Aging 13. Inadequately treated 21 hydroxylase deficiency 14. Thyroid dysfunction & hyperprolactinemia 15. Ovulation stimulation alone 16. Ovulation induction with or without Gn. RH agonists 17. ART

Luteal Phase Deficiency (LPD) Has been shown to occur: • During the postpartum period • With significant weight loss or exercise • In random cycles of normally menstruating women

Luteal phase deficiency (LPD) • Clinical significance: Controversy – Lack of a reliable test to diagnose – Only clinically relevant if it is consistently present in most cycles – Appears to be an association with infertility – Not been established as a cause of infertility • This report: Address controversies regarding the diagnosis & potential treatment of luteal inadequacy

MEDICAL CONDITIONS WITH POTENTIAL IMPACT ON LUTEAL PHASE FUNCTION

In women with hypothalamic amenorrhea • Abnormalities in Gn. RH, FSH, LH pulsatility ↓ Luteal estrogen & progesterone secretion • ↓ LH pulsatility abnormal progesterone secretion: – Problematic in ovulation induction cycles

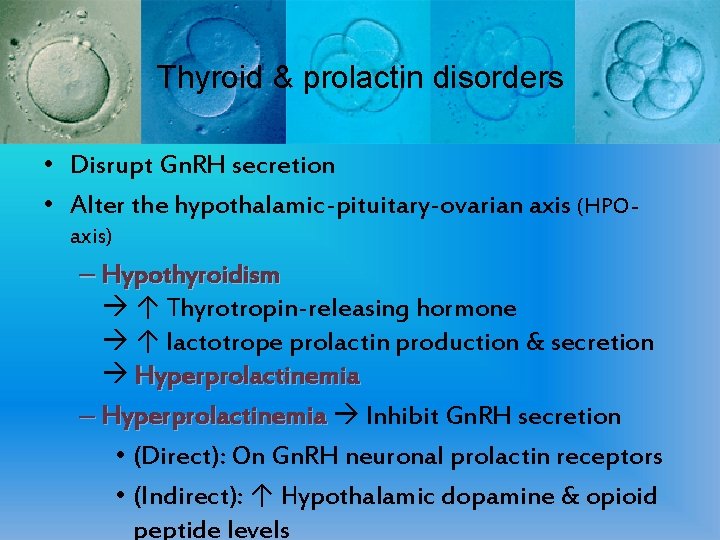
Thyroid & prolactin disorders • Disrupt Gn. RH secretion • Alter the hypothalamic-pituitary-ovarian axis (HPOaxis) – Hypothyroidism ↑ Thyrotropin-releasing hormone ↑ lactotrope prolactin production & secretion Hyperprolactinemia – Hyperprolactinemia Inhibit Gn. RH secretion • (Direct): On Gn. RH neuronal prolactin receptors • (Indirect): ↑ Hypothalamic dopamine & opioid peptide levels

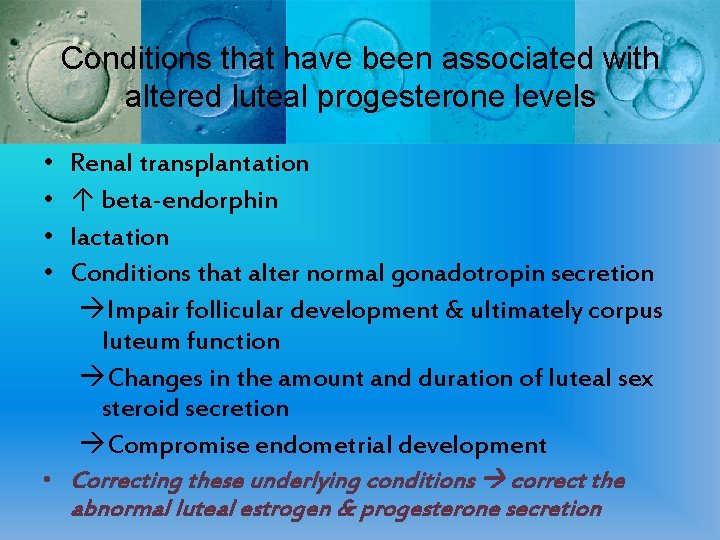
Conditions that have been associated with altered luteal progesterone levels • • Renal transplantation ↑ beta-endorphin lactation Conditions that alter normal gonadotropin secretion Impair follicular development & ultimately corpus luteum function Changes in the amount and duration of luteal sex steroid secretion Compromise endometrial development • Correcting these underlying conditions correct the abnormal luteal estrogen & progesterone secretion

Obesity • Negative impact: • ↓Fertility & ↑Pregnancy loss rate • Particularly evident in the morbidly obese • A recent study (Obese Normal weight women) • As with anorexic women • Alteration of LH pulsatility (↓Pulse amplitude) • ↓↓ luteal phase pregnanediol glucuronide (the major metabolite of progesterone) progesterone • The contribution to ↓ fecundity rates: rates Unknown

Ovarian Aging • Abnormalities in luteal phase function – Deficiencies in luteal phase progesterone (in early study) – Deficiencies in both luteal phase progesterone & estradiol metabolites (in recent study) • The contribution to ↓ pregnancy rates & ↑ loss rates: Unclear

Pathophysiology of luteal inadequacy • May include several different mechanisms that ultimately affect endometrial development

Short luteal phase • (LH peak ~ Onset of menstrual flow): Interval ≤ 8 days 11– 13 days Considered normal Follicular phase abnormalities • Low follicular FSH levels • Altered follicular FSH/LH ratios • Abnormal FSH & LH pulsatility ↓Luteal estrogen & progesterone levels • May occur in young healthy♀ with regular cycle length Clinical consequences: unclear

During cycles with IVF • Luteal phase may be abnormal • Cycle with Gn. RH agonists – Prolonged suppression of pituitary LH secretion (3 weeks after down regulation) – Luteal phase inadequacy and subfertility • Cycle with Gn. RH antagonists – Recovery of LH production from the pituitary is quite rapid following cessation – Still have significant reductions in pregnancy rates/ clear negative clinical impact in the luteal phase

During cycles with IVF • Hypothesis: In the stimulatory phase: High gonadotropin levels Suppress endogenous LH ↓ progesterone secretion & premature luteolysis ¶ In Superovulation & intrauterine insemination (SOIUI) cycles or Gonadotropin ovulation induction (OI) cycles for PCOS: – Adding Gn. RH agonists • Did not ↓ the pregnancy rate, luteal estrogen or progesterone levels • Did not alter endometrial dating

ARE THERE DIAGNOSTIC CRITERIA FOR INADEQUATE LUTEAL FUNCTION?

Diagnostic tests are influenced by and based upon the following physiologic observations: 1. Normal luteal phase length: 12– 14 days 2. [Progesterone] peak in non-pregnancy cycles: 6 ~ 8 days after ovulation 3. Progesterone is secreted in pulses 4. Endometrial response: A reflection of the follicular phase estrogen & the luteal phase estrogen & progesterone

5. Once implantation occurs, progesterone secretion by the corpus luteum: Dependent upon ↑ [h. CG] 6. Failure of [h. CG] ↑ directly causes corpus luteum failure ↓ progesterone levels

Methods proposed for diagnosing LPD • Basal body temperature (BBT) charting: • Inaccuracy, inconvenience, should be discouraged • Serum progesterone levels • Endometrial biopsy • Ovulation & adequate luteal length: • Urinary LH surge detection & Monitoring of luteal length

Progesterone Levels • Secreted in pulses (Reflect LH pulses) • Within 90 minutes: Fluctuate up to 8 -fold • After ovulation (- pregnancy): Peak 6 ~ 8 days – Determine peak progesterone levels Need determine the time of ovulation Urine LH false-positive LH surge

Progesterone Levels • During the luteal phase: No standard characterization • No minimum conc. ‘‘fertile’’ luteal function • Corpus luteum function in normal fertile women: varies from cycle to cycle Random levels: Not a valid clinical diagnostic tool to evaluate luteal phase adequacy

Progesterone Levels • Pregnancy h. CG Corpus luteum Progesterone ↓ Progesterone levels in early pregnancy: Nonviable or extra-uterine pregnancy Abnormal h. CG stimulation Should not be used to initiate therapy with exogenous progesterone

Endometrial Biopsy • Abnormalities of endometrial maturation: • Inadequate ovarian hormone secretion • Intrinsic endometrial abnormality • ‘‘Gold standard’’ to diagnose luteal inadequacy

Endometrial Biopsy • luteal inadequacy • Identify: Microscopic appearance of luteal phase endometrial development • Prevent: Normal implantation or early placental development • Associated with: Changes in steroid receptors, structural proteins, growth factors, cytokines, receptors, pinopodes • Normal luteal phase endometrial development: Clinically applicable criteria is complex & evolving

Endometrial Biopsy • For histologic endometrial dating Not a valid clinical diagnostic tool for • The identification of an infertile population • The diagnosis or treatment of LPD • Low progesterone levels Inadequate endometrial development ? • ↓ Progesterone to 3– 10 ng/m. L No evident impact with histological dating

Additional markers • Biochemical, morphological, or molecular markers of endometrial function To determined when or if the endometrium is receptive to implantation No marker validated in distinguishing normal fertile women from infertile women

Molecular markers of receptivity • In the subjects with ↓ progesterone replacement: Different endometrial protein expression Potentially more subtle deficiency • Remain experimental and are not valid clinical diagnostic tools

Summary • No reproducible, physiologically relevant, and clinically practical standard Diagnose LPD Distinguish fertile from infertile women • BBT, luteal progesterone levels, endometrial biopsy & other diagnostic studies Have not been established Performance of these tests cannot be recommended

IF DIAGNOSIS IS NOT POSSIBLE, IS TREATMENT FOR LUTEAL INADEQUACY EVER APPROPRIATE?

Treatment of potential luteal inadequacy • 1 st approach: Correction of any underlying condition (hypothalamic or thyroid dysfunction, hyperprolactinemia) • 2 nd: Empiric Treatment (based on limited reliable data) • Promote endometrial maturation • Enhance endometrial receptivity • Support implantation and development of an early pregnancy • Strategies: Progesterone, progesterone + estrogen, h. CG in the luteal phase, Ovulation induction with clomiphene or gonadotropins

Ovulation Induction • Improved pre-ovulatory follicular dynamics Should improve corpus luteum function ? Use of agents that induce ovulation Improved corpus luteum function & fertility outcomes • 1 st problem: Definition of luteal insufficiency • Surrogate endpoints: progesterone deficiency or out-of -phase endometrium • Poor fertility outcomes Surrogate endpoints Unsuccessful • 2 nd: ‘‘Ovulation induction strategies’’ improve fertility by inducing multiple ovulation and not by correcting LPD

Progesterone • Orally, Vaginally, Intramuscular route • Beneficial in natural, unstimulated cycles: no evidence • Only well-documented indication: For the improvement of ART outcomes in Gn. RH agonist or antagonist stimulation cycles

Progesterone supplementation • IM Progesterone Highest serum levels • Vaginal Progesterone ↑ Endometrial tissue levels • Oral progesterone Only ~10% of micronized progesterone is absorbed intact through GI tract ↓ Pregnancy rates Should not be used for luteal support • Should be administered until placental progesterone production is adequate (8– 10 weeks of gestation)

h. CG • In Gn. RH agonist/antagonist ART cycles • Luteal supplementation with h. CG Stimulates the ovaries (or corpora lutea) boost production of endogenous progesterone & estradiol ↑ Delivery rates ↓Spontaneous abortion rates ↑↑ Moderate or severe OHSS Low-dose h. CG (500 IU every other day) minimal risk of inducing OHSS

h. CG ü Clinical equivalence with intramuscular progesterone ü Higher incidence of side effects with Hcg Luteal progesterone is generally preferred in Gn. RH agonist/antagonist IVF cycles • Once pregnancy is established – Supplemental h. CG is not beneficial – RCT: h. CG for 1 st-trimester vaginal bleeding Miscarriage rate: 11% 12% (h. CG)

SUMMARY • Abnormal luteal function may occur as the result of a medical condition – e. g. , Elevated prolactin, abnormal thyroid function – Infertile women should be investigated and treated for these disorders and identified conditions • No diagnostic test for luteal phase insufficiency has been proven reliable in a clinical setting – BBT, luteal progesterone levels, endometrial biopsy, or other diagnostic studies – Performance cannot be recommended

• No treatment for luteal phase insufficiency has been shown to improve pregnancy outcomes in natural, unstimulated cycles • Luteal support after ART procedures (progesterone or h. CG) – Improves pregnancy outcomes – h. CG increases the risk of OHSS

• Not proven beneficial: – Progesterone or h. CG for luteal support: Once a pregnancy has been established – Progesterone in a non-ART cycle beyond the time of expected menses (i. e. , 2 weeks after ovulation)

CONCLUSIONS • Progesterone is important for the process of implantation and early embryonic development • LPD, as an independent entity causing infertility, has not been proven

THANK YOU FOR LISTENING
 Dr brendan miller
Dr brendan miller Anovulatory cycle
Anovulatory cycle Luteal phase support in art
Luteal phase support in art Short luteal phase progesterone
Short luteal phase progesterone Short luteal phase
Short luteal phase Clinical relevance
Clinical relevance Foliküler faz nedir
Foliküler faz nedir Hiptalamus
Hiptalamus Csce 441
Csce 441 Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Hplc reverse phase vs normal phase
Hplc reverse phase vs normal phase Power formula three phase
Power formula three phase Mobile phase and stationary phase
Mobile phase and stationary phase Phase to phase voltage
Phase to phase voltage Detectors used in hplc
Detectors used in hplc Stationary phase
Stationary phase Relevance feedback example
Relevance feedback example Rigor relevance and relationships
Rigor relevance and relationships Connective words
Connective words Rigor relevance and relationships in action
Rigor relevance and relationships in action Cs 276
Cs 276 Example of relevance
Example of relevance Formal and informal fallacy
Formal and informal fallacy Relevance of entrepreneurship
Relevance of entrepreneurship Note making advantages
Note making advantages Esg relevance score
Esg relevance score Importance of aromatic plants
Importance of aromatic plants Counsell 5 rs
Counsell 5 rs Relevance lost the rise and fall of management accounting
Relevance lost the rise and fall of management accounting Relationships before rigor
Relationships before rigor Relevance information retrieval
Relevance information retrieval Cs 276
Cs 276 Types of relevance
Types of relevance Pseudo relevance feedback
Pseudo relevance feedback Relevance theory in pragmatics
Relevance theory in pragmatics Maxim of relevance
Maxim of relevance The perceived relevance or importance of an ethical issue
The perceived relevance or importance of an ethical issue Relevance information retrieval
Relevance information retrieval Rigor relevance framework
Rigor relevance framework Directive principles of state policy notes
Directive principles of state policy notes Improving search relevance
Improving search relevance 401 relevance
401 relevance Fallacies of relevance examples
Fallacies of relevance examples Relevance in education
Relevance in education