Tec and EcoCement Update Tec Eco Masonry Products










- Slides: 61

Tec and Eco-Cement Update Tec. Eco Masonry Products utilising Tec and Tec. Eco eco-cements I will have to race over some slides but the presentation is always downloadable from the Tec. Eco web site if you missed something. John Harrison B. Sc. B. Ec. FCPA. Presentation downloadable from www. tececo. com 1

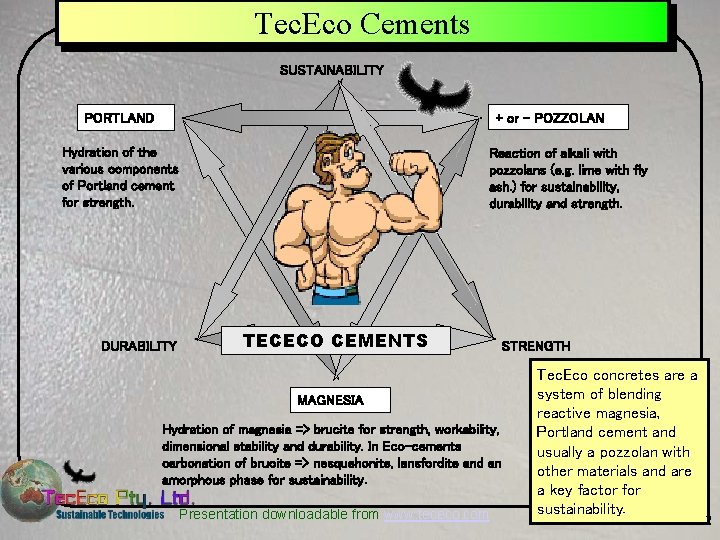
Tec. Eco Cements SUSTAINABILITY PORTLAND + or - POZZOLAN Hydration of the various components of Portland cement for strength. DURABILITY Reaction of alkali with pozzolans (e. g. lime with fly ash. ) for sustainability, durability and strength. TECECO CEMENTS STRENGTH MAGNESIA Hydration of magnesia => brucite for strength, workability, dimensional stability and durability. In Eco-cements carbonation of brucite => nesquehonite, lansfordite and an amorphous phase for sustainability. Presentation downloadable from www. tececo. com Tec. Eco concretes are a system of blending reactive magnesia, Portland cement and usually a pozzolan with other materials and are a key factor for sustainability. 2

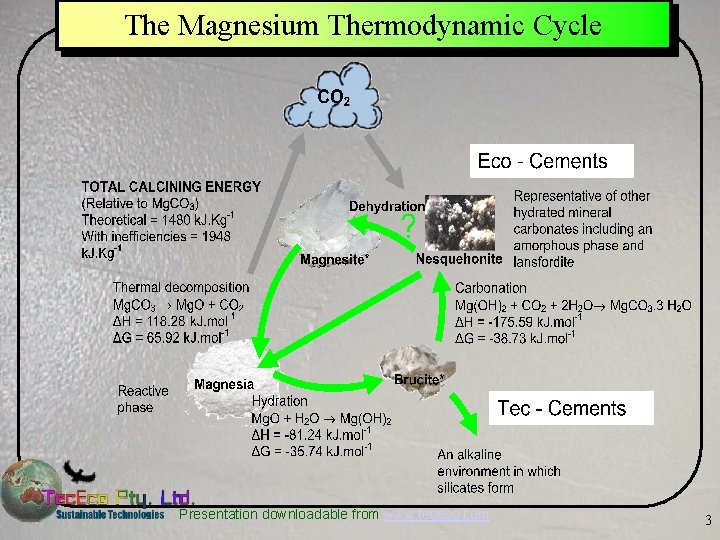
The Magnesium Thermodynamic Cycle Presentation downloadable from www. tececo. com 3

Tec. Eco Cement Sustainability Ü Tec. Eco technology will be pivotal in bringing about sustainability in the built environment. – The CO 2 released by calcined carbonates used to make binders can be captured using Tec. Eco kiln technology. – Tec-Cements Develop Significant Early Strength even with Added Supplementary Materials. • Around 25 = 30% less total binder is required for the same strength. – Eco-cements carbonate sequestering CO 2 – Both tec and eco=cements provide a benign low p. H environment for hosting large quantities of waste overcoming problems of: • Using acids to etch plastics so they bond with concretes. • sulphates from plasterboard etc. ending up in recycled construction materials. • heavy metals and other contaminants. • delayed reactivity e. g. ASR with glass cullet • Durability issues Presentation downloadable from www. tececo. com 4

Tec. Eco Formulations Ü Tec-cements (Low Mg. O) – contain more Portland cement than reactive magnesia. Reactive magnesia hydrates in the same rate order as Portland cement forming Brucite which uses up water reducing the voids: paste ratio, increasing density and possibly raising the short term p. H. – Reactions with pozzolans are more affective. After all the Portlandite has been consumed Brucite controls the long term p. H which is lower and due to it’s low solubility, mobility and reactivity results in greater durability. – Other benefits include improvements in density, strength and rheology, reduced permeability and shrinkage and the use of a wider range of aggregates many of which are potentially wastes without reaction problems. Ü Eco-cements (High Mg. O) – contain more reactive magnesia than in tec-cements. Brucite in porous materials carbonates forming stronger fibrous mineral carbonates and therefore presenting huge opportunities for waste utilisation and sequestration. Ü Enviro-cements (High Mg. O) – contain similar ratios of Mg. O and OPC to eco-cements but in non porous concretes brucite does not carbonate readily. – Higher proportions of magnesia are most suited to toxic and hazardous waste immobilisation and when durability is required. Strength is not developed quickly nor to the same extent. Presentation downloadable from www. tececo. com 5

Tec. Eco Cement Technology ÜPortlandite (Ca(OH)2) is too soluble, mobile and reactive. – It carbonates, reacts with Cl- and SO 4 - and being soluble can act as an electrolyte. ÜTec. Eco generally (but not always) remove Portlandite using the pozzolanic reaction and ÜTec. Eco add reactive magnesia – which hydrates forming brucite which is another alkali, but much less soluble, mobile or reactive than Portlandite. ÜIn Eco-cements brucite carbonates The consequences of need to be considered Presentation downloadable from www. tececo. com 6

Why Add Reactive Magnesia? Ü To maintain the long term stability of CSH. – Maintains alkalinity preventing the reduction in Ca/Si ratio. Ü To remove water. – Reactive magnesia consumes water as it hydrates to possibly hydrated forms of brucite. Ü To reduce shrinkage. – The consequences of putting brucite through the matrix of a concrete in the first place need to be considered. Ü To make concretes more durable Ü Because significant quantities of carbonates are produced in porous substrates which are affective binders. Reactive Mg. O is a new tool to be understood with profound affects on most properties Presentation downloadable from www. tececo. com 7

What is Reactive Mg. O? or Lattice Energy Destroys a Myth Ü Magnesia, provided it is reactive rather than “dead burned” (or high density, crystalline periclase type), can be beneficially added to cements in excess of the amount of 5 mass% generally considered as the maximum allowable by standards prevalent in concrete dogma. – Reactive magnesia is essentially amorphous magnesia with low lattice energy. – It is produced at low temperatures and finely ground, and – will completely hydrate in the same time order as the minerals contained in most hydraulic cements. Ü Dead burned magnesia and lime have high lattice energies – Crystalline magnesium oxide or periclase has a calculated lattice energy of 3795 Kj mol-1 which must be overcome for it to go into solution or for reaction to occur. – Dead burned magnesia is much less expansive than dead burned lime (Ramachandran V. S. , Concrete Science, Heydon & Son Ltd. 1981, p 358 -360 ) Presentation downloadable from www. tececo. com 8

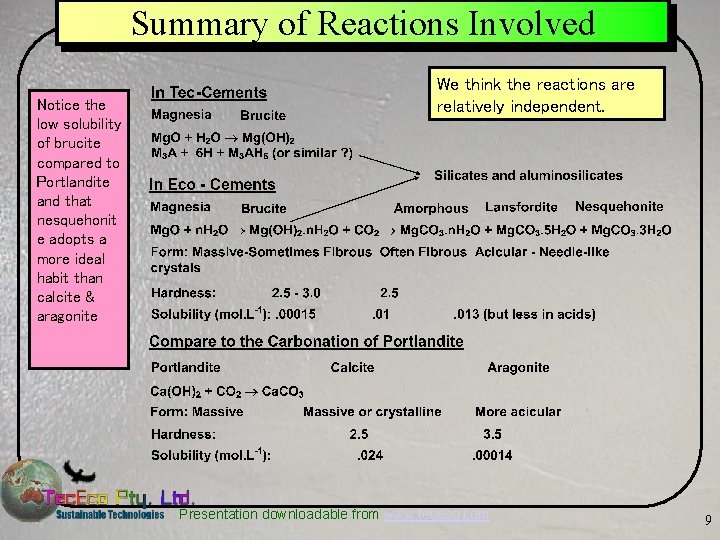
Summary of Reactions Involved Notice the low solubility of brucite compared to Portlandite and that nesquehonit e adopts a more ideal habit than calcite & aragonite We think the reactions are relatively independent. Presentation downloadable from www. tececo. com 9

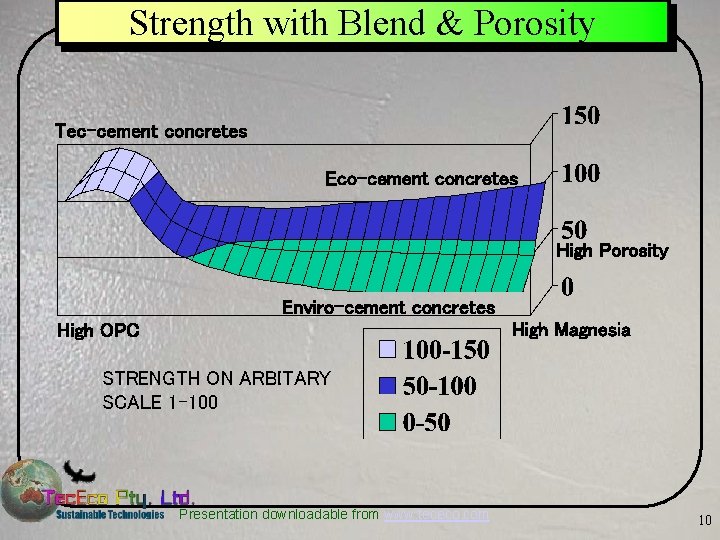
Strength with Blend & Porosity Tec-cement concretes Eco-cement concretes High Porosity Enviro-cement concretes High Magnesia High OPC STRENGTH ON ARBITARY SCALE 1 -100 Presentation downloadable from www. tececo. com 10

Eco-Cements Ü Eco-cements are similar but potentially superior to lime mortars because: – The calcination phase of the magnesium thermodynamic cycle takes place at a much lower temperature and is therefore more efficient. – Magnesium minerals are generally more fibrous and acicular than calcium minerals and hence add microstructural strength. – Water forms part of the binder minerals that forming making the cement component go further. In terms of binder produced for starting material in cement, eco-cements are nearly six times more efficient. – Magnesium hydroxide in particular and to some extent the carbonates are less reactive and mobile and thus much more durable. Presentation downloadable from www. tececo. com 11

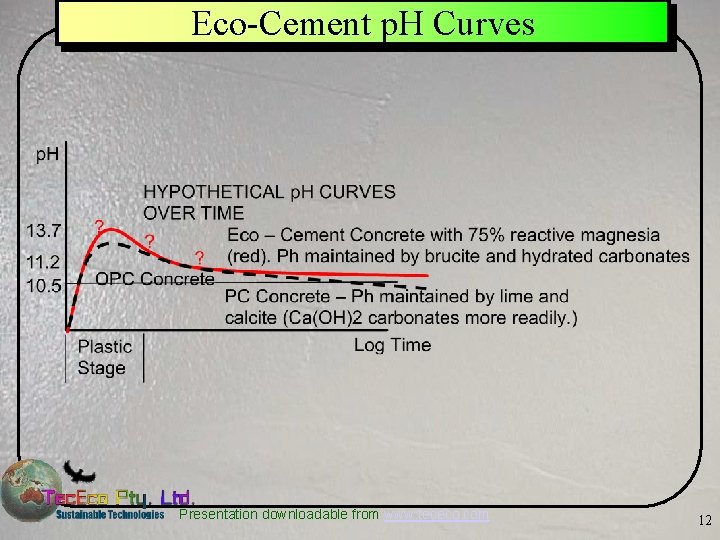
Eco-Cement p. H Curves Presentation downloadable from www. tececo. com 12

Eco-Cement Strength Development Ü Eco-cements gain early strength from the hydration of PC. Ü Later strength comes from the carbonation of brucite forming an amorphous phase, lansfordite and nesquehonite. Ü Strength gain in eco-cements is mainly microstructural because of – More ideal particle packing (Brucite particles at 4 -5 micron are under half the size of cement grains. ) – The natural fibrous and acicular shape of magnesium carbonate minerals which tend to lock together. Ü More binder is formed than with calcium – Total volumentric expansion from magnesium oxide to lansfordite is for example 473 volume %. Presentation downloadable from www. tececo. com 13

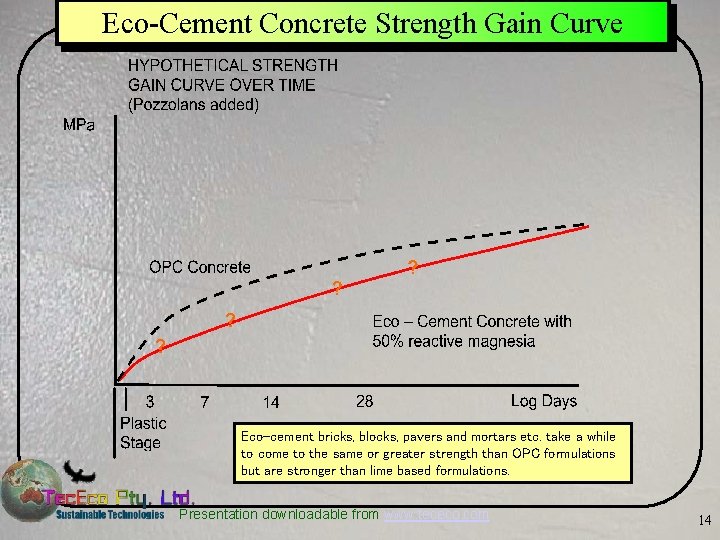
Eco-Cement Concrete Strength Gain Curve Eco-cement bricks, blocks, pavers and mortars etc. take a while to come to the same or greater strength than OPC formulations but are stronger than lime based formulations. Presentation downloadable from www. tececo. com 14

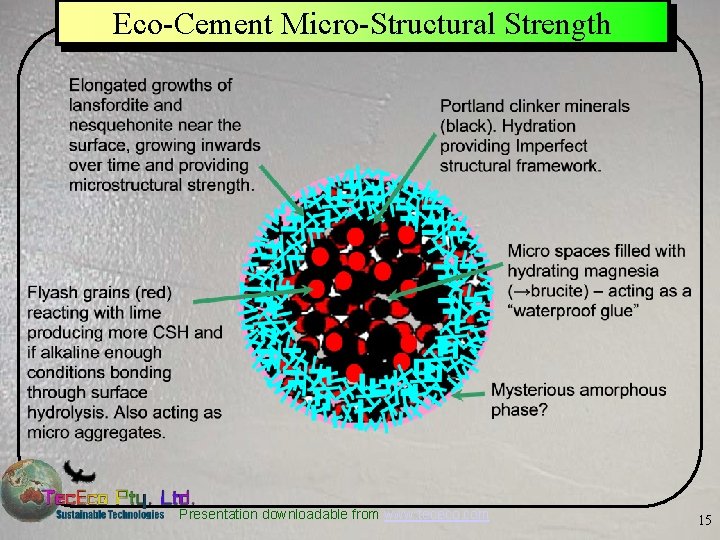
Eco-Cement Micro-Structural Strength Presentation downloadable from www. tececo. com 15

Carbonation Ü Because magnesium has a low molecular weight, proportionally a greater amount of CO 2 is captured. Ü Carbonation results in significant sequestration because of the shear volumes involved. Ü Carbonation adds strength. Ü Carbonates are the stable phases of both calcium and magnesium. Ü The formation of carbonates lowers the p. H of concretes compromising the stability of the passive oxide coating on steel. Ü Some steel reinforced structural concrete could be replaced with fibre reinforced porous carbonated concrete. Presentation downloadable from www. tececo. com 16

Chemistry of Carbonation Ü There a number of carbonates of magnesium. The main ones appear to be an amorphous phase, lansfordite and nesquehonite. Ü The carbonation of magnesium hydroxide does not proceed as readily as that of calcium hydroxide. – Gor Brucite to nesquehonite = - 38. 73 k. J. mol-1 – Compare to Gor Portlandite to calcite = -64. 62 k. J. mol-1 Ü The dehydration of nesquehonite to form magnesite is not favoured by simple thermodynamics but may occur in the long term under the right conditions. Ü Gor nesquehonite to magnesite = 8. 56 k. J. mol-1 – But kinetically driven by desiccation during drying. Ü Reactive magnesia can carbonate in dry conditions – so keep bags sealed! Ü For a full discussion of thermodynamics see our technical documents. Tec. Eco technical documents on the web cover the important aspects of carbonation. Presentation downloadable from www. tececo. com 17

Ramifications of Carbonation Ü Magnesium Carbonates. – The magnesium carbonates that form at the surface of tec – cement concretes expand significantly thereby sealing off further carbonation. – Lansfordite and nesquehonite are stronger and more acid resistant than calcite or aragonite. – The curing of eco-cements in a moist - dry alternating environment seems to encourage carbonation. Ü Portland Cement Concretes – Carbonation proceeds relatively rapidly at the surface. Vaterite followed by Aragonite and Calcite is the principal product and lowers the p. H to around 8. 2 Presentation downloadable from www. tececo. com 18

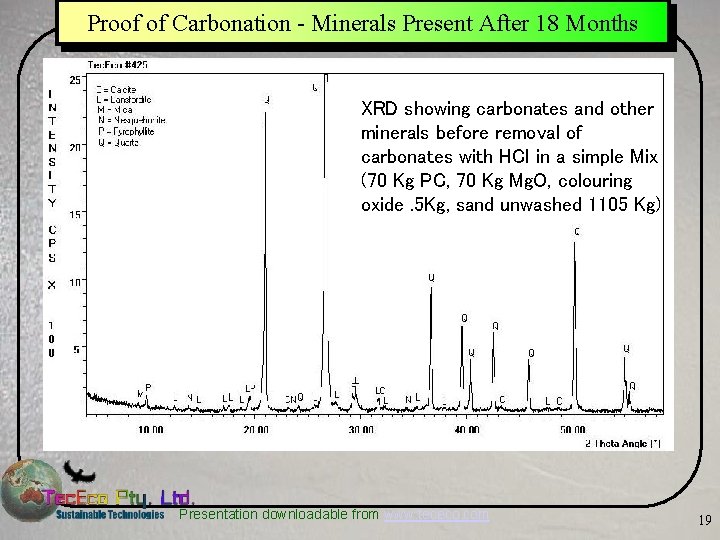
Proof of Carbonation - Minerals Present After 18 Months XRD showing carbonates and other minerals before removal of carbonates with HCl in a simple Mix (70 Kg PC, 70 Kg Mg. O, colouring oxide. 5 Kg, sand unwashed 1105 Kg) Presentation downloadable from www. tececo. com 19

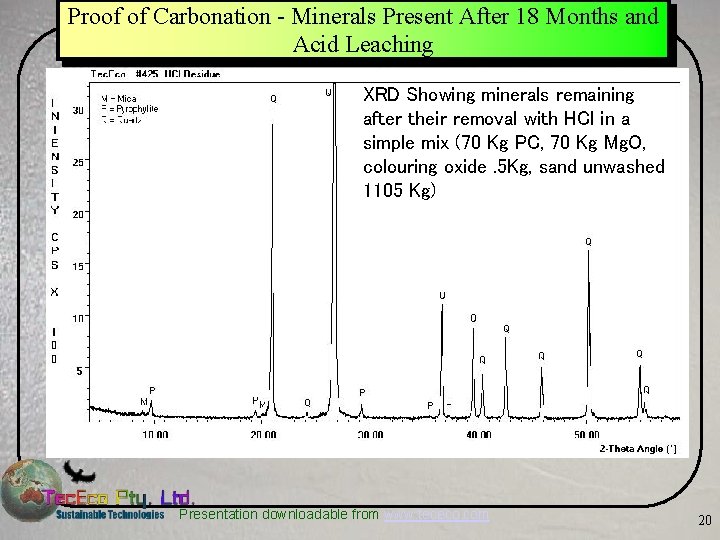
Proof of Carbonation - Minerals Present After 18 Months and Acid Leaching XRD Showing minerals remaining after their removal with HCl in a simple mix (70 Kg PC, 70 Kg Mg. O, colouring oxide. 5 Kg, sand unwashed 1105 Kg) Presentation downloadable from www. tececo. com 20

Tec. Eco Binders - Solving Waste Problems Ü There are huge volumes of concrete produced annually ( 2 tonnes person per year. ) Ü An important objective should be to make cementitous composites that can utilise wastes. Ü Tec. Eco cements provide a benign environment suitable for waste immobilisation Ü Many wastes such as fly ash, sawdust , shredded plastics etc. can improve a property or properties of the cementitious composite. There are huge materials flows in both wastes and building and construction. Tec. Eco technology will lead the world in the race to incorporate wastes in cementitous composites Presentation downloadable from www. tececo. com 21

Tec. Eco Binders - Solving Waste Problems (2) Ü Tec. Eco cementitious composites represent a cost affective option for both use and immobilisation of waste. – Lower reactivity • less water • lower p. H – Reduced solubility of heavy metals • less mobile salts – Greater durability. • Denser. • Impermeable (tec-cements). • Dimensionally more stable with less shrinkage and cracking. – Homogenous. – No bleed water. Tec. Eco Technology Converting Waste to Resource Presentation downloadable from www. tececo. com 22

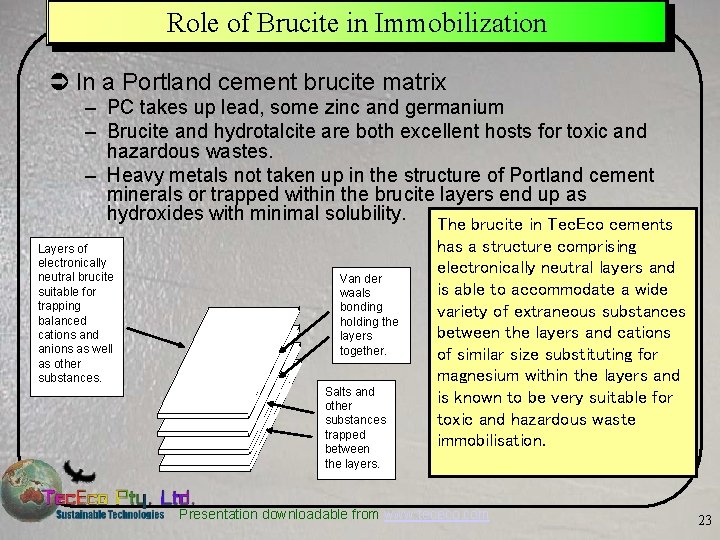
Role of Brucite in Immobilization Ü In a Portland cement brucite matrix – PC takes up lead, some zinc and germanium – Brucite and hydrotalcite are both excellent hosts for toxic and hazardous wastes. – Heavy metals not taken up in the structure of Portland cement minerals or trapped within the brucite layers end up as hydroxides with minimal solubility. The brucite in Tec. Eco cements Layers of electronically neutral brucite suitable for trapping balanced cations and anions as well as other substances. Van der waals bonding holding the layers together. Salts and other substances trapped between the layers. has a structure comprising electronically neutral layers and is able to accommodate a wide variety of extraneous substances between the layers and cations of similar size substituting for magnesium within the layers and is known to be very suitable for toxic and hazardous waste immobilisation. Presentation downloadable from www. tececo. com 23

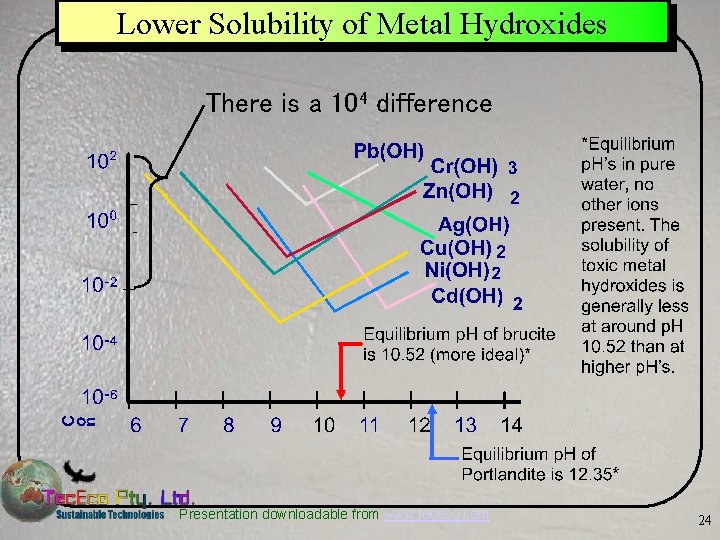
Lower Solubility of Metal Hydroxides There is a 104 difference Presentation downloadable from www. tececo. com 24

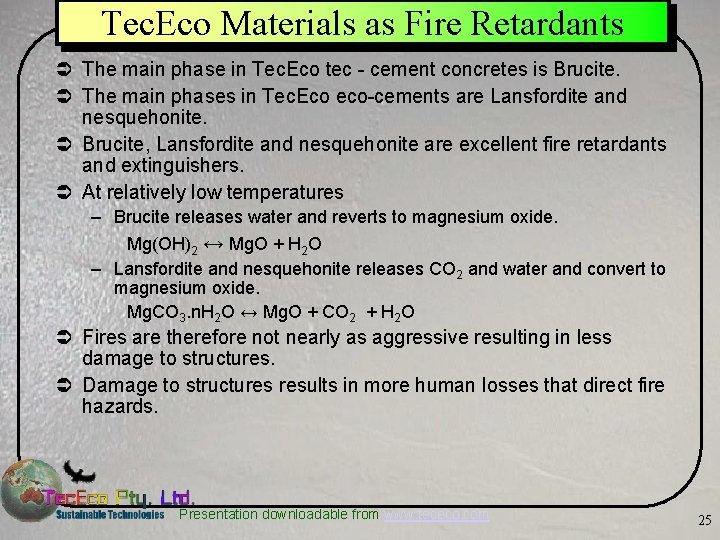
Tec. Eco Materials as Fire Retardants Ü The main phase in Tec. Eco tec - cement concretes is Brucite. Ü The main phases in Tec. Eco eco-cements are Lansfordite and nesquehonite. Ü Brucite, Lansfordite and nesquehonite are excellent fire retardants and extinguishers. Ü At relatively low temperatures – Brucite releases water and reverts to magnesium oxide. Mg(OH)2 ↔ Mg. O + H 2 O – Lansfordite and nesquehonite releases CO 2 and water and convert to magnesium oxide. Mg. CO 3. n. H 2 O ↔ Mg. O + CO 2 + H 2 O Ü Fires are therefore not nearly as aggressive resulting in less damage to structures. Ü Damage to structures results in more human losses that direct fire hazards. Presentation downloadable from www. tececo. com 25

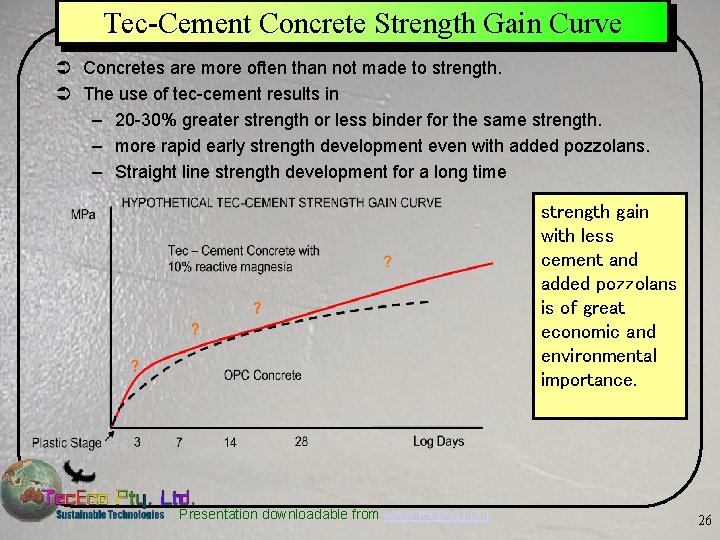
Tec-Cement Concrete Strength Gain Curve Ü Concretes are more often than not made to strength. Ü The use of tec-cement results in – 20 -30% greater strength or less binder for the same strength. – more rapid early strength development even with added pozzolans. – Straight line strength development for a long time strength gain with less cement and added pozzolans is of great economic and environmental importance. Presentation downloadable from www. tececo. com 26

Reasons for Strength Development in Tec-Cements. Ü Reactive magnesia requires considerable water to hydrate resulting in: – Denser, less permeable concrete. – A significantly lower voids/paste ratio. Ü Higher early p. H initiating more effective silicification reactions? – The Ca(OH)2 normally lost in bleed water is used internally for reaction with pozzolans. – Super saturation of alkalis caused by the removal of water? Ü Micro-structural strength due to particle packing (Magnesia particles at 4 -5 micron are a little over ½ the size of cement grains. ) Ü Slow release of water from hydrated Mg(OH)2. n. H 2 O supplying H 2 O for more complete hydration of C 2 S and C 3 S? Ü Formation of Mg. Al hydrates? Similar to flash set in concrete but slower? ? Presentation downloadable from www. tececo. com 27

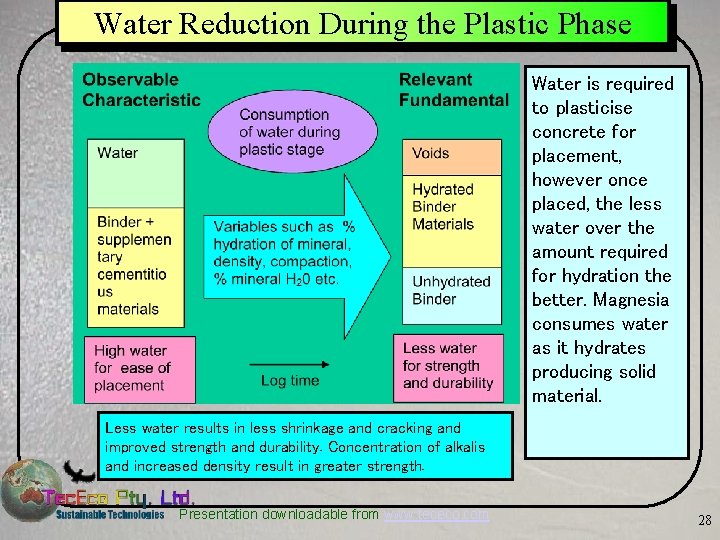
Water Reduction During the Plastic Phase Water is required to plasticise concrete for placement, however once placed, the less water over the amount required for hydration the better. Magnesia consumes water as it hydrates producing solid material. Less water results in less shrinkage and cracking and improved strength and durability. Concentration of alkalis and increased density result in greater strength. Presentation downloadable from www. tececo. com 28

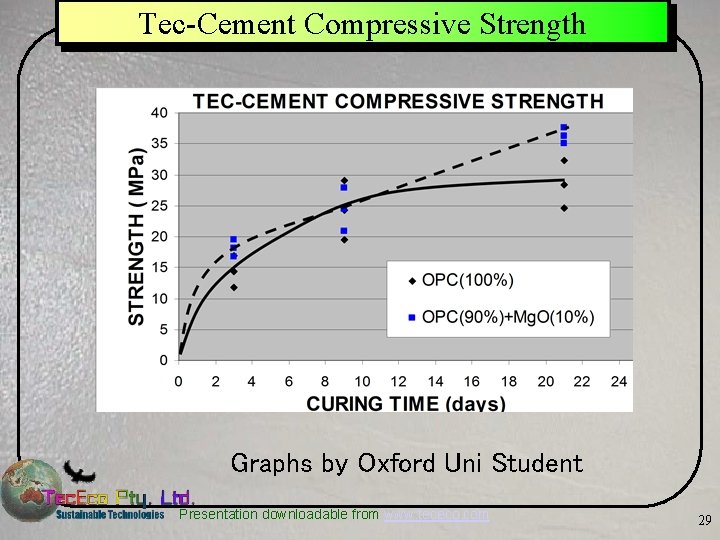
Tec-Cement Compressive Strength Graphs by Oxford Uni Student Presentation downloadable from www. tececo. com 29

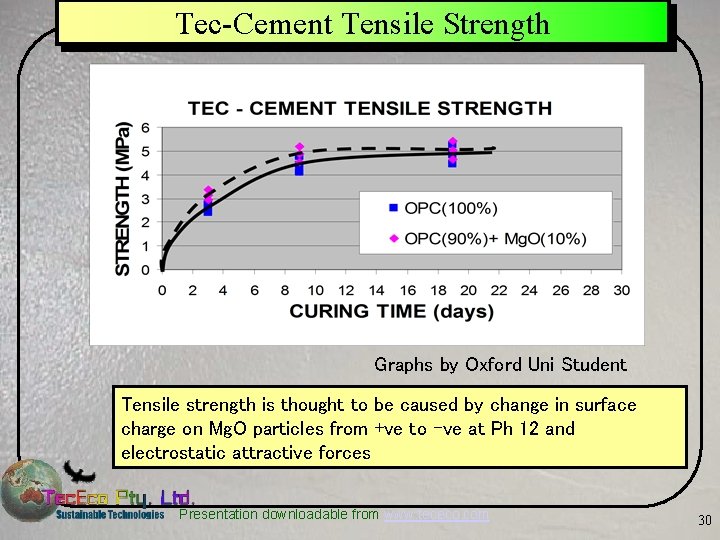
Tec-Cement Tensile Strength Graphs by Oxford Uni Student Tensile strength is thought to be caused by change in surface charge on Mg. O particles from +ve to –ve at Ph 12 and electrostatic attractive forces Presentation downloadable from www. tececo. com 30

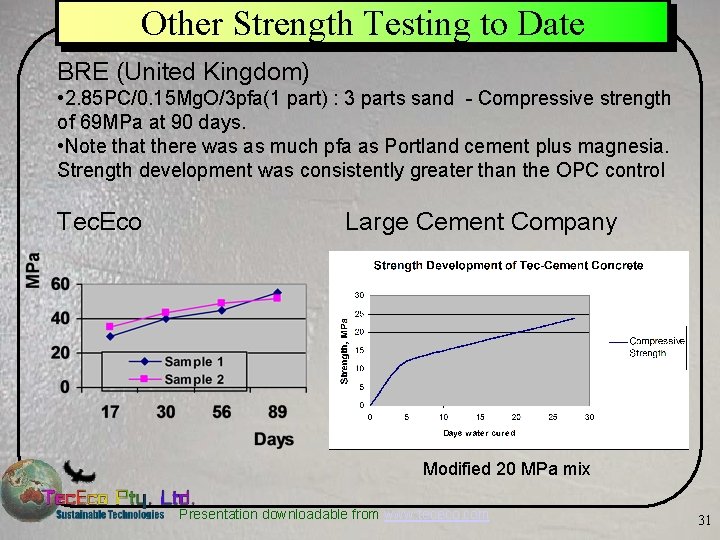
Other Strength Testing to Date BRE (United Kingdom) • 2. 85 PC/0. 15 Mg. O/3 pfa(1 part) : 3 parts sand - Compressive strength of 69 MPa at 90 days. • Note that there was as much pfa as Portland cement plus magnesia. Strength development was consistently greater than the OPC control Tec. Eco Large Cement Company Modified 20 MPa mix Presentation downloadable from www. tececo. com 31

Increased Density – Reduced Permeability Ü Concretes have a high percentage (around 18% - 25%) of voids. Ü On hydration magnesia expands 116. 9 % filling voids and surrounding hydrating cement grains and compensates for the shrinkage of Portland cement. Ü Brucite is 44. 65 mass% water. Ü Lower voids: paste ratios than water: binder ratios result in little or no bleed water less permeability and greater density. – Compare the affect to that of vacuum dewatering. Presentation downloadable from www. tececo. com 32

Reduced Permeability Ü As bleed water exits ordinary Portland cement concretes it creates an interconnected pore structure that remains in concrete allowing the entry of aggressive agents such as SO 4 --, Cland CO 2 Ü Tec. Eco tec - cement concretes are a closed system. They do not bleed as excess water is consumed by the hydration of magnesia. Ü Consequences: – Tec - cement concretes tend to dry from within, are denser and less permeable and therefore stronger more durable and more waterproof. Cement powder is not lost near the surfaces. – Tec-cements have a higher salt resistance and less corrosion of steel etc. Presentation downloadable from www. tececo. com 33

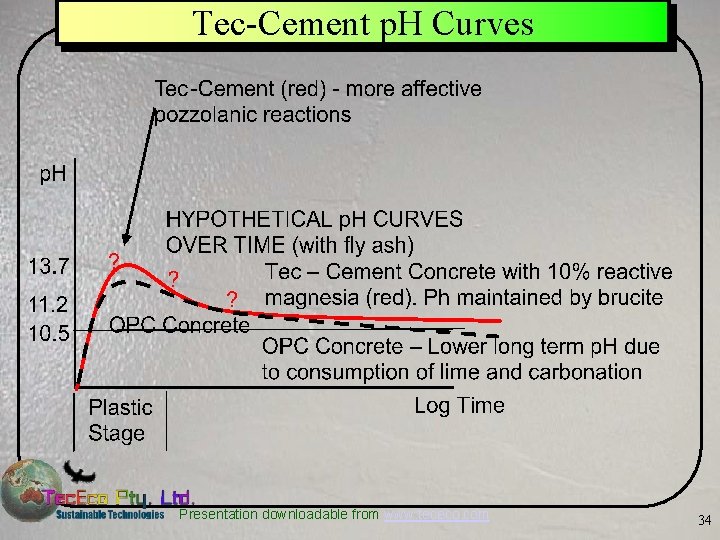
Tec-Cement p. H Curves Presentation downloadable from www. tececo. com 34

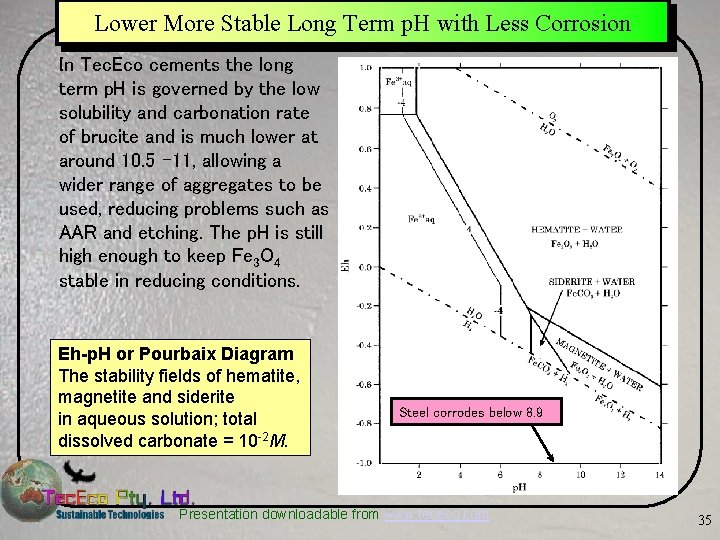
Lower More Stable Long Term p. H with Less Corrosion In Tec. Eco cements the long term p. H is governed by the low solubility and carbonation rate of brucite and is much lower at around 10. 5 -11, allowing a wider range of aggregates to be used, reducing problems such as AAR and etching. The p. H is still high enough to keep Fe 3 O 4 stable in reducing conditions. Eh-p. H or Pourbaix Diagram The stability fields of hematite, magnetite and siderite in aqueous solution; total dissolved carbonate = 10 -2 M. Steel corrodes below 8. 9 Presentation downloadable from www. tececo. com 35

Reduced Steel Corrosion Ü Steel remains protected with a passive oxide coating of Fe 3 O 4 above p. H 8. 9. – A p. H of over 8. 9 is maintained by the equilibrium Mg(OH)2 ↔ Mg++ + 2 OHfor much longer than the p. H maintained by Ca(OH)2 because: – Brucite does not react as readily as Portlandite resulting in reduced carbonation rates and reactions with salts. Ü Concrete with brucite in it is denser and carbonation is expansive, sealing the surface preventing further access by moisture, CO 2 and salts. Ü Brucite is less soluble and traps salts as it forms resulting in less ionic transport to complete a circuit for electrolysis and less corrosion. Ü Free chlorides and sulfates originally in cement and aggregates are bound by magnesium – Magnesium oxychlorides or oxysulfates are formed. ( Compatible phases in hydraulic binders that are stable provided the concrete is dense and water kept out. ) Presentation downloadable from www. tececo. com 36

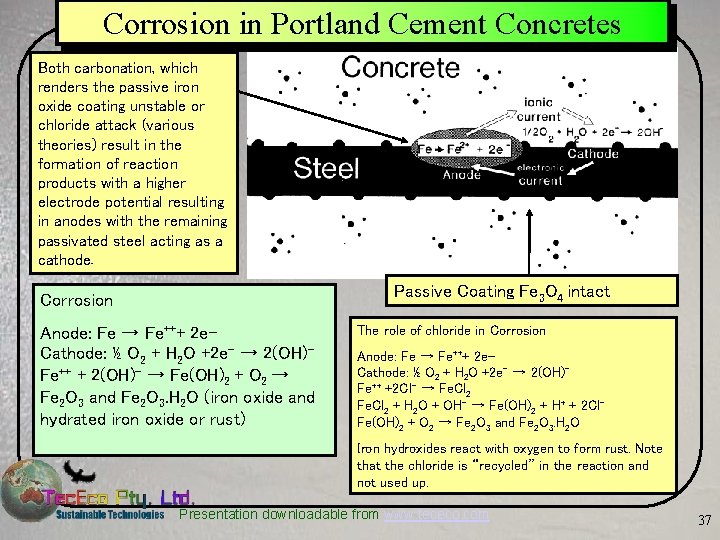
Corrosion in Portland Cement Concretes Both carbonation, which renders the passive iron oxide coating unstable or chloride attack (various theories) result in the formation of reaction products with a higher electrode potential resulting in anodes with the remaining passivated steel acting as a cathode. Passive Coating Fe 3 O 4 intact Corrosion Anode: Fe → Fe+++ 2 e. Cathode: ½ O 2 + H 2 O +2 e- → 2(OH)Fe++ + 2(OH)- → Fe(OH)2 + O 2 → Fe 2 O 3 and Fe 2 O 3. H 2 O (iron oxide and hydrated iron oxide or rust) The role of chloride in Corrosion Anode: Fe → Fe+++ 2 e. Cathode: ½ O 2 + H 2 O +2 e- → 2(OH)Fe++ +2 Cl- → Fe. Cl 2 + H 2 O + OH- → Fe(OH)2 + H+ + 2 Cl. Fe(OH)2 + O 2 → Fe 2 O 3 and Fe 2 O 3. H 2 O Iron hydroxides react with oxygen to form rust. Note that the chloride is “recycled” in the reaction and not used up. Presentation downloadable from www. tececo. com 37

Reduced Delayed Reactions Ü A wide range of delayed reactions can occur in Portland cement based concretes – Delayed alkali silica and alkali carbonate reactions – The delayed formation of ettringite and thaumasite – Delayed hydration of minerals such as dead burned lime and magnesia. Ü Delayed reactions cause dimensional distress and possible failure. Presentation downloadable from www. tececo. com 38

Reduced Delayed Reactions (2) Ü Delayed reactions do not appear to occur to the same extent in Tec. Eco cements. – A lower long term p. H results in reduced reactivity after the plastic stage. – Potentially reactive ions are trapped in the structure of brucite. – Ordinary Portland cement concretes can take years to dry out however the reactive magnesia in Tec-cement concretes consumes unbound water from the pores inside concrete, probably holding it for slow release to extended hydration reactions of Ca silicates. – Magnesia dries concrete out from the inside. Reactions do not occur without water. Presentation downloadable from www. tececo. com 39

Durability - Reduced Salt & Acid Attack Ü Brucite has always played a protective role during salt attack. Putting it in the matrix of concretes in introduces considerable durability. Ü Brucite does not react with salts because it is a least 5 orders of magnitude less soluble, mobile or reactive. – Ksp brucite = 1. 8 X 10 -11 – Ksp Portlandite = 5. 5 X 10 -6 Ü Tec. Eco cements are more acid resistant than Portland cement – This is because of the relatively high acid resistance (? ) of Lansfordite and nesquehonite compared to calcite or aragonite Presentation downloadable from www. tececo. com 40

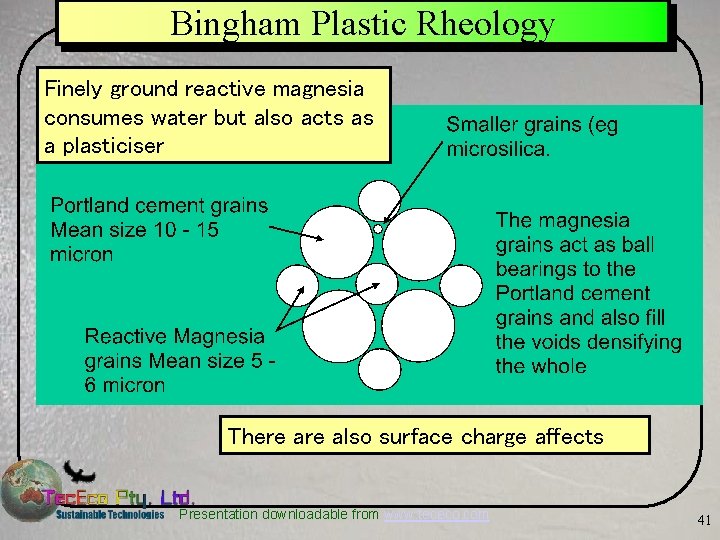
Bingham Plastic Rheology Finely ground reactive magnesia consumes water but also acts as a plasticiser There also surface charge affects Presentation downloadable from www. tececo. com 41

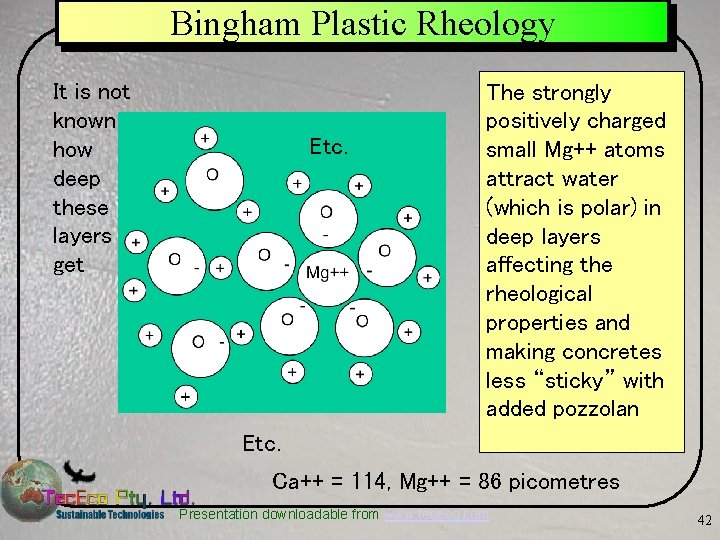
Bingham Plastic Rheology It is not known how deep these layers get Etc. The strongly positively charged small Mg++ atoms attract water (which is polar) in deep layers affecting the rheological properties and making concretes less “sticky” with added pozzolan Etc. Ca++ = 114, Mg++ = 86 picometres Presentation downloadable from www. tececo. com 42

Rheology Ü Tec. Eco concretes and mortars are: – Very homogenous and do not segregate easily. They exhibit good adhesion and have a shear thinning property. – Exhibit Bingham plastic qualities and react well to energy input. – Have good workability. Ü Tec. Eco concretes with the same water/binder ratio have a lower slump but greater plasticity and workability. Ü A range of pumpable composites with Bingham plastic properties will be required in the future as buildings will be “printed. ” Presentation downloadable from www. tececo. com 43

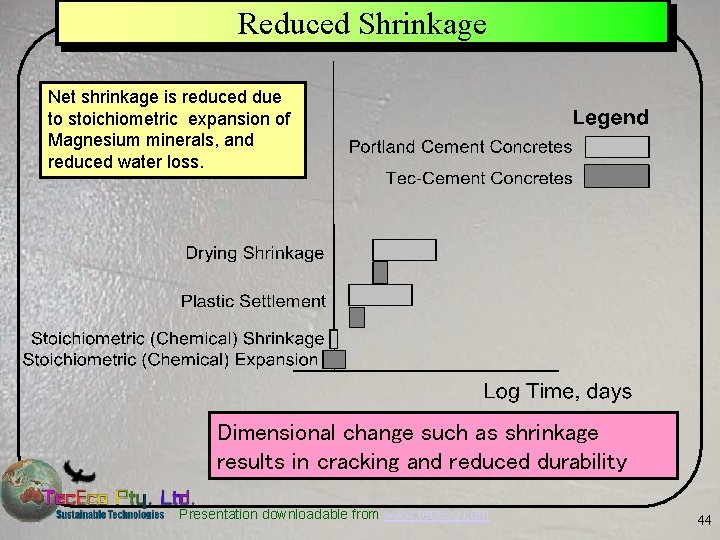
Reduced Shrinkage Net shrinkage is reduced due to stoichiometric expansion of Magnesium minerals, and reduced water loss. Dimensional change such as shrinkage results in cracking and reduced durability Presentation downloadable from www. tececo. com 44

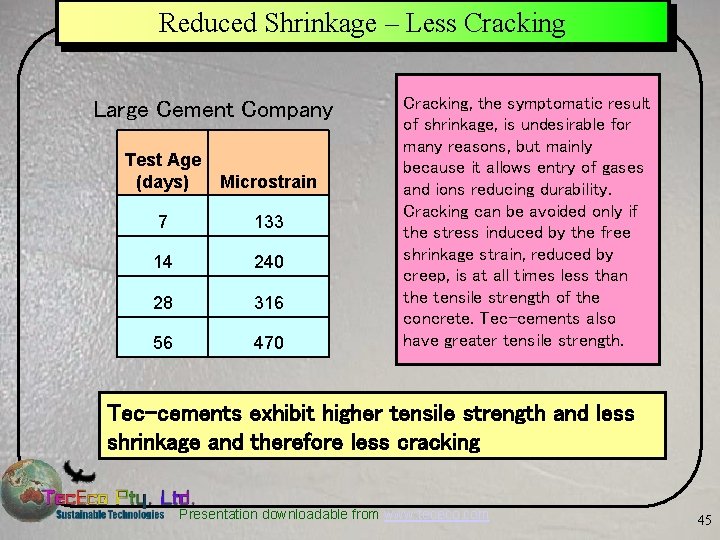
Reduced Shrinkage – Less Cracking Large Cement Company Test Age (days) Microstrain 7 133 14 240 28 316 56 470 Cracking, the symptomatic result of shrinkage, is undesirable for many reasons, but mainly because it allows entry of gases and ions reducing durability. Cracking can be avoided only if the stress induced by the free shrinkage strain, reduced by creep, is at all times less than the tensile strength of the concrete. Tec-cements also have greater tensile strength. Tec-cements exhibit higher tensile strength and less shrinkage and therefore less cracking Presentation downloadable from www. tececo. com 45

Volume Changes on Hydration ÜWhen magnesia hydrates it expands: Mg. O (s) + H 2 O (l) ↔ Mg(OH)2. n. H 2 O (s) 40. 31 + 18. 0 ↔ 58. 3 (minimum) molar mass 11. 2 + liquid ↔ 24. 3 (minimum) molar volumes Ü Up to 116. 96% solidus expansion depending on whether the water is coming from stoichiometric mix water, bleed water or from outside the system. In practice less as the water comes from mix and bleed water. The molar volume (L. mol-1)is equal to the molar mass (g. mol-1) divided by the density (g. L-1). Presentation downloadable from www. tececo. com 46

Volume Changes on Carbonation Ü Consider what happens when Portlandite carbonates: Ca(OH)2 + CO 2 Ca. CO 3 74. 08 + 44. 01 ↔ 100 molar mass 33. 22 + gas ↔ 36. 93 molar volumes – Slight expansion. But shrinkage from surface water loss Ü Compared to brucite forming nesquehonite as it carbonates: Mg(OH)2 + CO 2 Mg. CO 3. 3 H 2 O 58. 31 + 44. 01 ↔ 138. 32 molar mass 24. 29 + gas ↔ 74. 77 molar volumes – 307 % expansion (less water volume reduction) and densification of the surface preventing further ingress of CO 2 and carbonation. Self sealing? The molar volume (L. mol-1)is equal to the molar mass (g. mol-1) divided by the density (g. L-1). Presentation downloadable from www. tececo. com 47

Dimensionally Control Over Concretes During Curing? Ü Portland cement concretes shrink around. 05%. Over the long term much more (>. 1%). – Mainly due to plastic and drying shrinkage. Ü The use of some wastes as aggregates causes shrinkage e. g. wood waste in masonry units, thin panels etc. Ü By varying the amount and form of magnesia added dimensional control can be achieved. Presentation downloadable from www. tececo. com 48

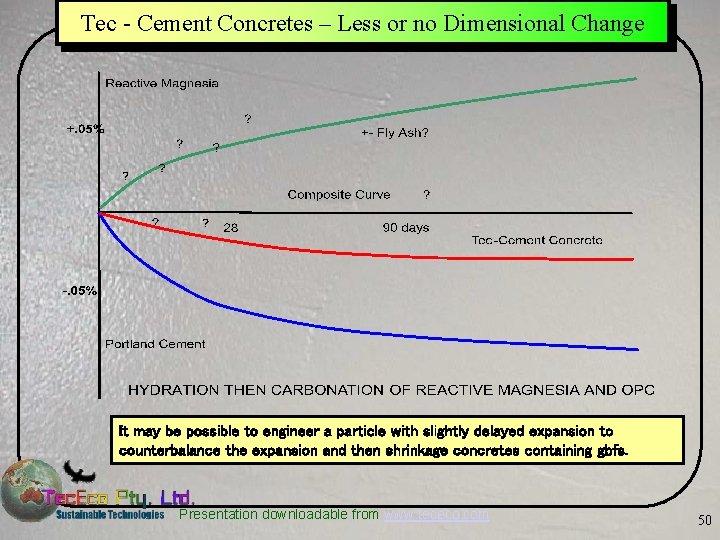
Tec. Eco Cement Concretes –Dimensional Control Ü Combined – Hydration and Carbonation can be manipulated to be close to neutral. – So far we have not observed significant shrinkage in Tec. Eco tec - cement concretes (5% -10% substitution OPC) also containing fly ash. – At some ratio, thought to be around 10% reactive magnesia and 90% PC volume changes are optimised as higher additions of Mg. O reduce strength. – The water lost by Portland cement as it shrinks is used by reactive magnesia as it hydrates also reducing shrinkage. Presentation downloadable from www. tececo. com 49

Tec - Cement Concretes – Less or no Dimensional Change It may be possible to engineer a particle with slightly delayed expansion to counterbalance the expansion and then shrinkage concretes containing gbfs. Presentation downloadable from www. tececo. com 50

Less Freeze - Thaw Problems Ü Denser concretes do not let water in. Ü Brucite will to a certain extent take up internal stresses Ü When magnesia hydrates it expands into the pores left around hydrating cement grains: Mg. O (s) + H 2 O (l) ↔ Mg(OH)2 (s) 40. 31 + 18. 0 ↔ 58. 3 molar mass 11. 2 + 18. 0 ↔ 24. 3 molar volumes 39. 20 ↔ 24. 3 molar volumes 38% air voids are created in space that was occupied by magnesia and water! Ü Air entrainment can also be used as in conventional concretes Ü Tec. Eco concretes are not attacked by the salts used on roads Presentation downloadable from www. tececo. com 51

Tec. Eco Cement Implementation Summary Presentation downloadable from www. tececo. com 52

High Performance-Lower Construction Costs Ü Less binders (OPC + magnesia) for the same strength. Ü Faster strength gain even with added pozzolans. Ü Elimination of shrinkage reducing Foolproof associated costs. Concrete? Ü Tolerance and consumption of water. Ü Reduction in bleed water enables finishing of lower floors whilst upper floors still being poured and increases pumpability. Ü Cheaper binders as less energy required Ü Increased durability will result in lower costs/energies/emissions due to less frequent replacement. Ü Because reactive magnesia is also an excellent plasticiser, other costly additives are not required for this purpose. Ü A wider range of aggregates can be utilised without problems reducing transport and other costs/energies/emissions. Presentation downloadable from www. tececo. com 53

Tec. Eco Concretes - Lower Construction Costs (2) Ü Ü Ü Homogenous, do not segregate with pumping or work. Easier placement and better finishing. Reduced or eliminated carbon taxes. Eco-cements can to a certain extent be recycled. Tec. Eco cements utilise wastes many of which improve properties. Improvements in insulating capacity and other properties will result in greater utility. Ü Products utilising Tec. Eco cements such as masonry and precast products can in most cases utilise conventional equipment and have superior properties. Ü A high proportion of brucite compared to Portlandite is water and of Lansfordite and nesquehonite compared to calcite is CO 2. – Every mass unit of Tec. Eco cements therefore produces a greater volume of built environment than Portland other calcium based cements. Less need therefore be used reducing costs/energy/emissions. Presentation downloadable from www. tececo. com 54

Relevance to the Masonry Industry Ü The Canadian masonry industry is ideally placed to take advantage of the Kyoto protocol to solve the world’s global warming problem as the country: – Making bricks, blocks, pavers and mortars using tec or eco-cements in Canada would help the country meet its Kyoto objectives and together with the raw materials required provide a new export. Canada: Ü Canada: – Is close by countries that are big emitters (Europe, the US) – Has abundant Mg minerals suitable for a silicate reactor process to sequester CO 2 from concentrated sources such as power stations etc. – Has abundant non fossil fuel energy (hydro, wind) to power Tec. Eco kilns – Is close to markets that could use Mg carbonate products with associated carbon credits Presentation downloadable from www. tececo. com 55

Summary Ü Simple, smart and sustainable? – Tec. Eco cement technology has resulted in potential solutions to a number of problems with Portland other cements including shrinkage, durability and corrosion and the immobilisation of many problem wastes and will provides a range of more sustainable building materials. üClimate Change üPollution üDurability üCorrosion üStrength üDelayed Reactions üPlacement , Finishing üRheology üShrinkage üCarbon Taxes Ü The right technology at the right time? – Tec. Eco cement technology addresses important triple bottom line issues solving major global problems with positive economic and social outcomes. “There is a way to make our city streets as green as the Amazon rainforest” Fred Pearce New Scientist Magazine Presentation downloadable from www. tececo. com 56

Tec. Eco Doing Things Presentation downloadable from www. tececo. com 57

The Use of Eco-Cements for Building Earthship Brighton By Taus Larsen, (Architect, Low Carbon Network Ltd. ) The Low Carbon Network (www. lowcarbon. co. uk) was established to raise awareness of the links between buildings, the working and living patterns they create, and global warming and aims to initiate change through the application of innovative ideas and approaches to construction. England’s first Earthship is currently under construction in southern England outside Brighton at Stanmer Park and Tec. Eco technologies have been used for the floors and some walling. Earthships are exemplars of low-carbon design, construction and living and were invented and developed in the USA by Mike Reynolds over 20 years of practical building exploration. They are autonomous earth-sheltered buildings independent from mains electricity, water and waste systems and have little or no utility costs. For information about the Earthship Brighton and other projects please go to the Tec. Eco web site. Presentation downloadable from www. tececo. com 58

Repair of Concrete Blocks. Clifton Surf Club The Clifton Surf Life Saving Club was built by first pouring footings, On the footings block walls were erected and then at a later date concrete was laid in between. As the ground underneath the footings was sandy, wet most of the time and full of salts it was a recipe for disaster. Predictably the salty water rose up through the footings and then through the blocks and where the water evaporated there was strong efflorescence, pitting, loss of material and damage. The Tec. Eco solution was to make up a formulation of eco-cement mortar which we doctored with some special chemicals to prevent the rise of any more moisture and salt. The solution worked well and appears to have stopped the problem. Presentation downloadable from www. tececo. com 59

Mike Burdon’s Murdunna Works Mike Burdon, Builder and Plumber. I work for a council interested in sutainability and have been involved with Tec. Eco since around 2001 in a private capacity helping with large scale testing of Tec. Eco tec-cements at our shack. I am interested in the potentially superior strength development and sustainability aspects. To date we have poured two slabs, footings, part of a launching ramp and some tilt up panels using formulations and materials supplied by John Harrison of Tec. Eco. I believe that research into the new Tec. Eco cements essential as overall I have found: 1. The rheological performance even without plasticizer was excellent. As testimony to this the contractors on the site commented on how easy the concrete was to place and finish. 2. We tested the Tec. Eco formulations with a hired concrete pump and found it extremely easy to pump and place. Once in position it appeared to “gel up” quickly allowing stepping for a foundation to a brick wall. 3. Strength gain was more rapid than with Portland cement controls from the same premix plant and continued for longer. 4. The surfaces of the concrete appeared to be particularly hard and I put this down to the fact that much less bleeding was observed than would be expected with a Portland cement only formulation Presentation downloadable from www. tececo. com 60

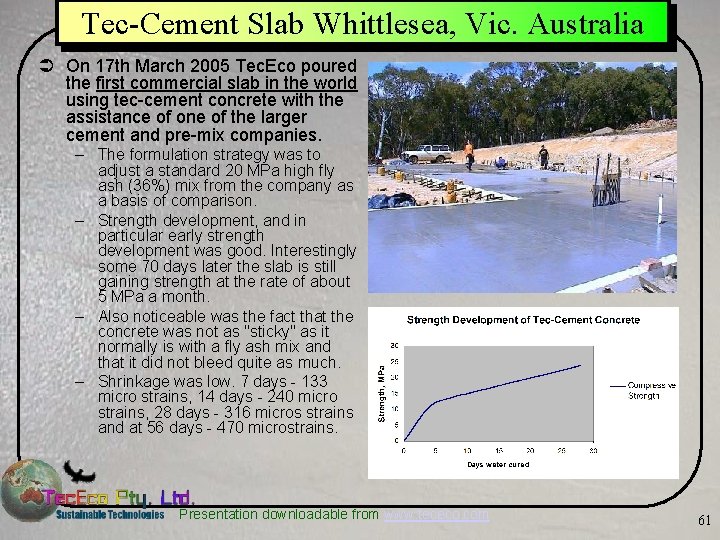
Tec-Cement Slab Whittlesea, Vic. Australia Ü On 17 th March 2005 Tec. Eco poured the first commercial slab in the world using tec-cement concrete with the assistance of one of the larger cement and pre-mix companies. – The formulation strategy was to adjust a standard 20 MPa high fly ash (36%) mix from the company as a basis of comparison. – Strength development, and in particular early strength development was good. Interestingly some 70 days later the slab is still gaining strength at the rate of about 5 MPa a month. – Also noticeable was the fact that the concrete was not as "sticky" as it normally is with a fly ash mix and that it did not bleed quite as much. – Shrinkage was low. 7 days - 133 micro strains, 14 days - 240 micro strains, 28 days - 316 micros strains and at 56 days - 470 microstrains. Presentation downloadable from www. tececo. com 61