Stoichiometry Mass of Component Elements Percent Composition Mass






- Slides: 21

Stoichiometry

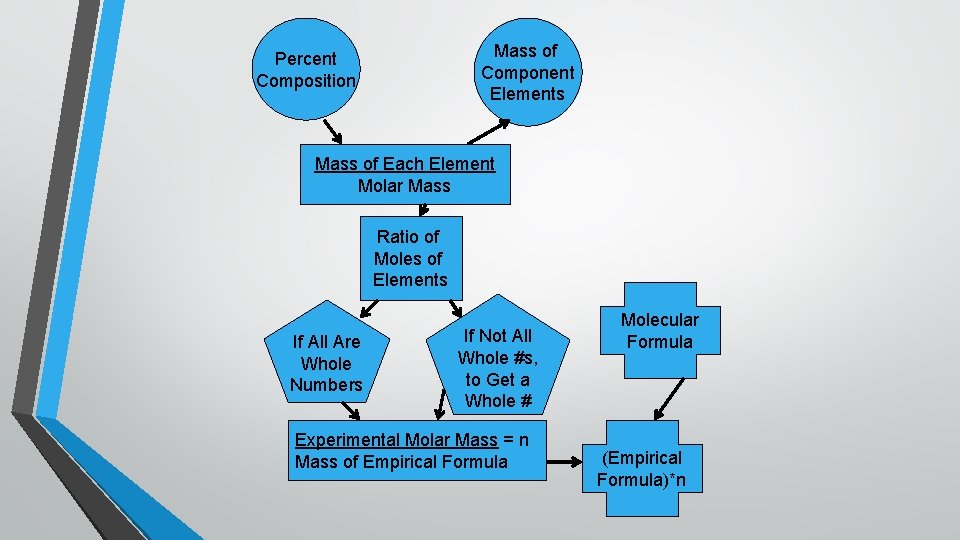
Mass of Component Elements Percent Composition Mass of Each Element Molar Mass Ratio of Moles of Elements If All Are Whole Numbers If Not All Whole #s, to Get a Whole # Experimental Molar Mass = n Mass of Empirical Formula Molecular Formula (Empirical Formula)*n

Stoichiometry • Chemical reactions will stop when one of the reactants is used up (unless it is a reversible reaction) • Stoichiometry is the tool to measuring the amount of reactants needed to have them all used up in the end • The study of quantitative relationships between amounts of reactants used and products formed by chemical reactions

Stoichiometry • It is based on the Law of Conservation of Mass, which was introduced by Antoine Lavoisier in the 18 th century • Recall: Law of Conservation of Mass/Energy • Mass is neither created nor destroyed in a chemical reaction, but preserved throughout • Chemical bonds in reactants are broken and new chemical bonds form to produce products, but the amount of matter/mass/energy present at the end of the reaction is the same as was present at the beginning

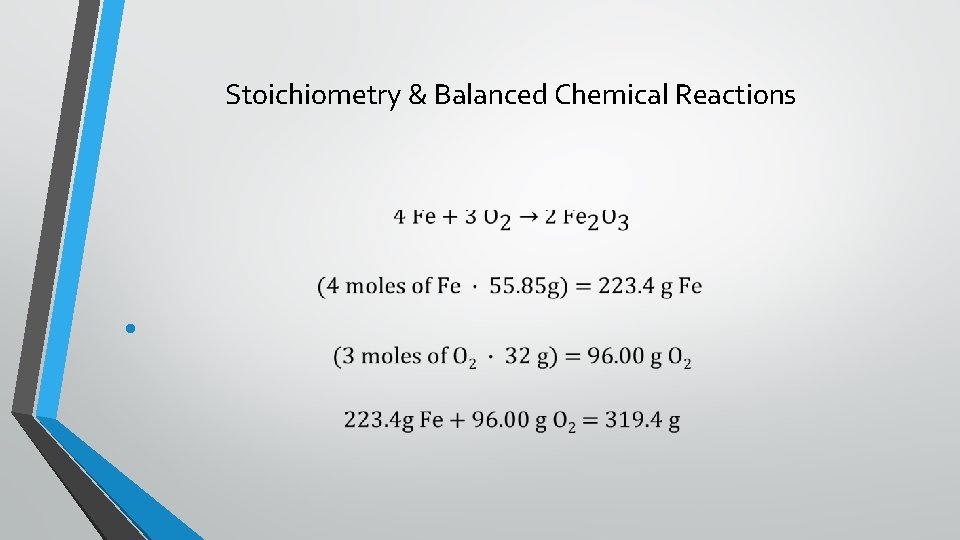
Stoichiometry & Balanced Chemical Reactions •

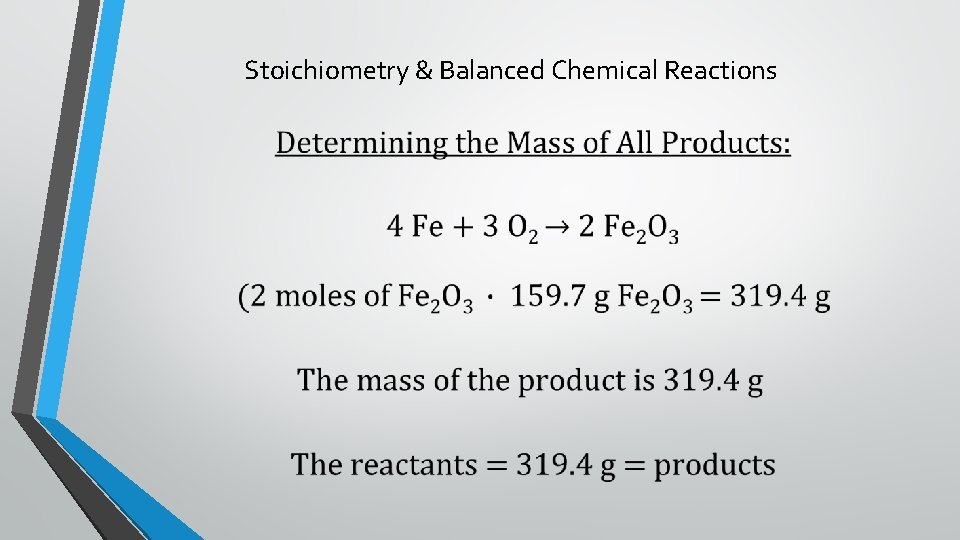
Stoichiometry & Balanced Chemical Reactions

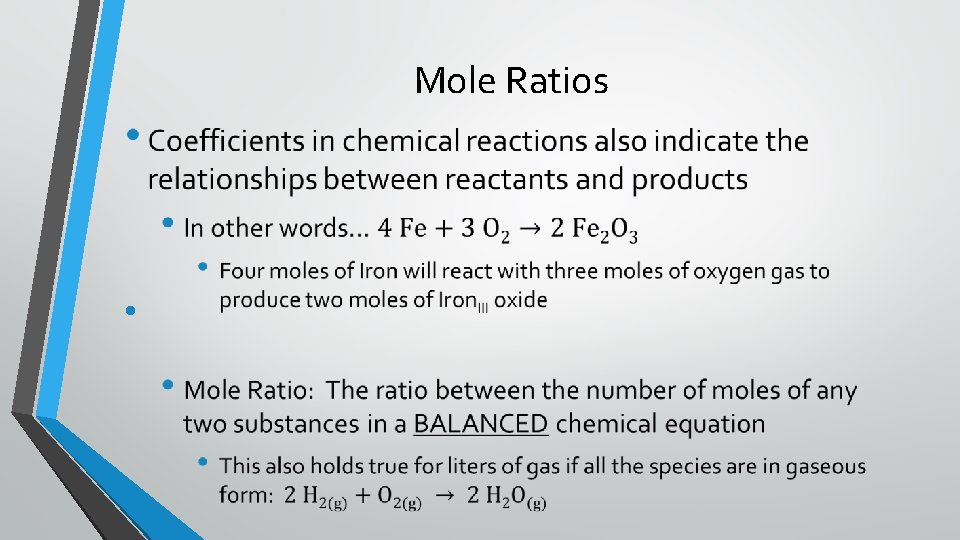
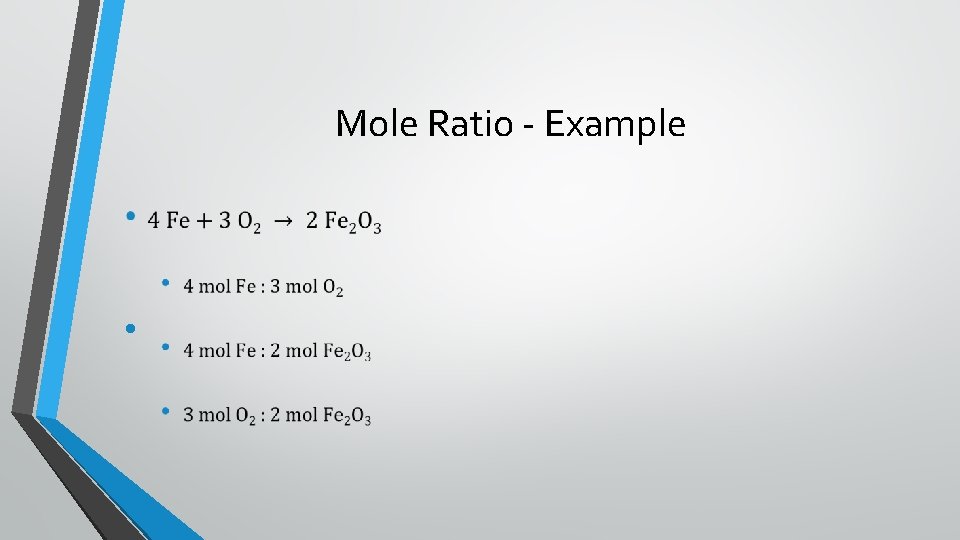
Mole Ratios •

Mole Ratio - Example •

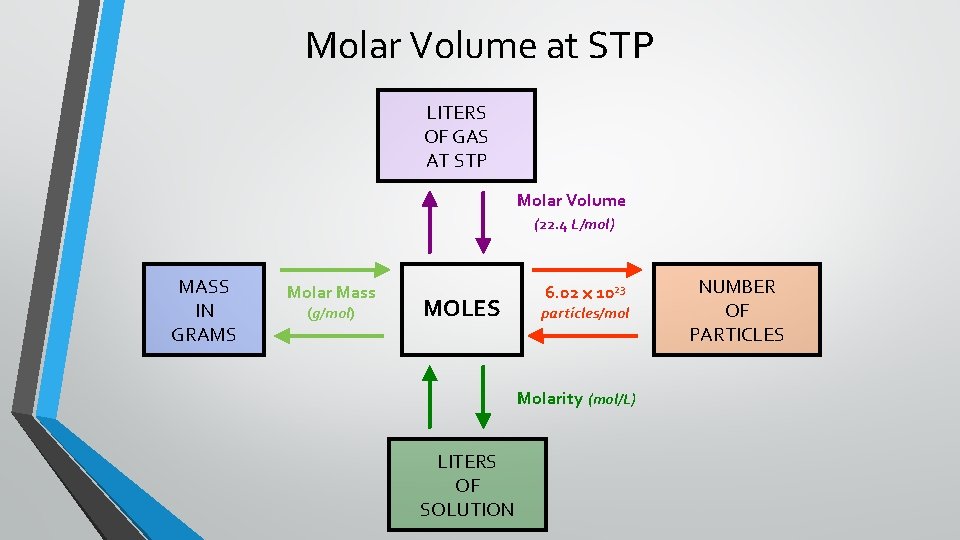
Molar Volume at STP LITERS OF GAS AT STP Molar Volume (22. 4 L/mol) MASS IN GRAMS Molar Mass (g/mol) MOLES 6. 02 1023 particles/mol Molarity (mol/L) LITERS OF SOLUTION NUMBER OF PARTICLES

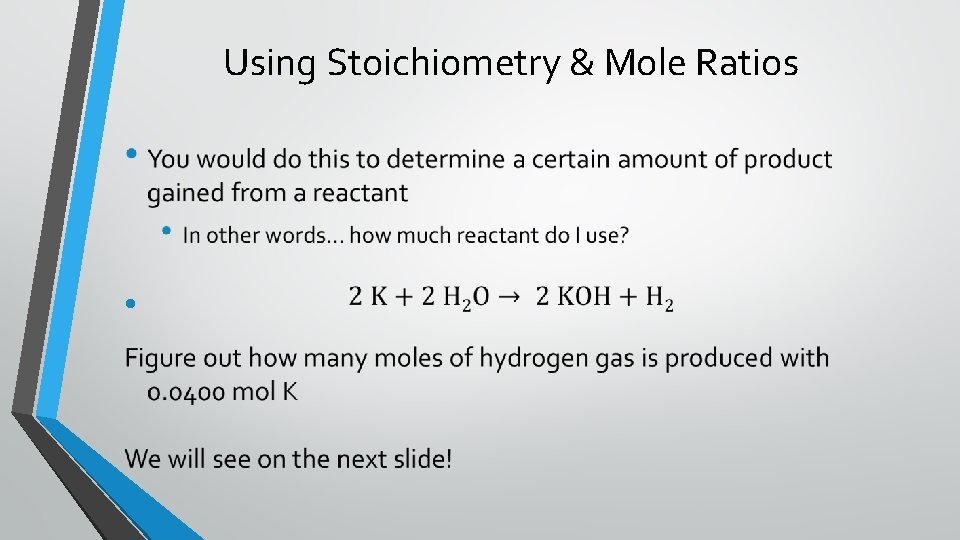
Using Stoichiometry & Mole Ratios •

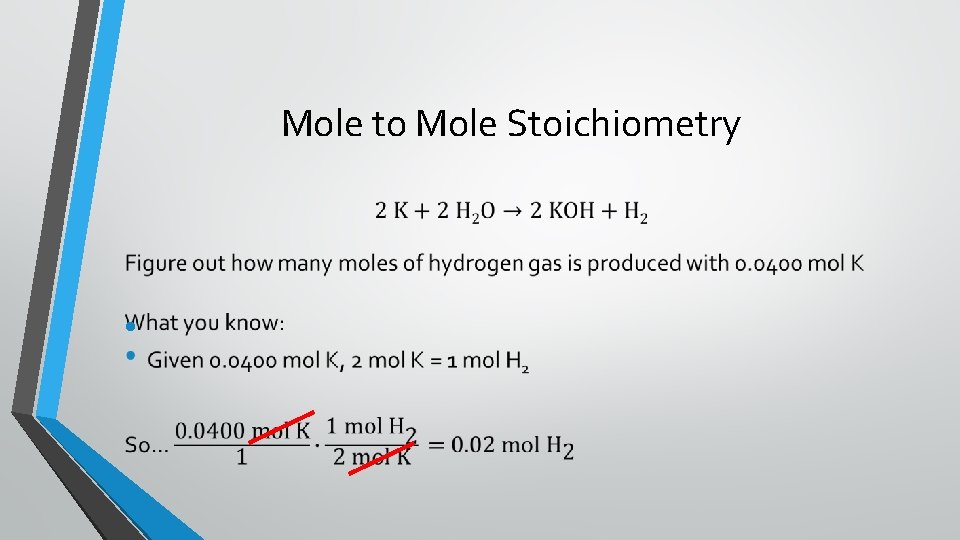
Mole to Mole Stoichiometry •

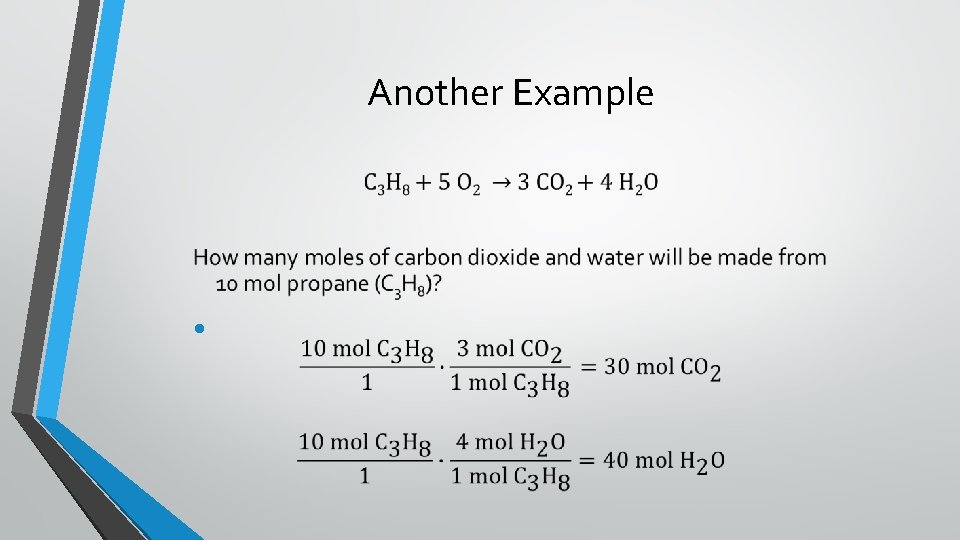
Another Example •

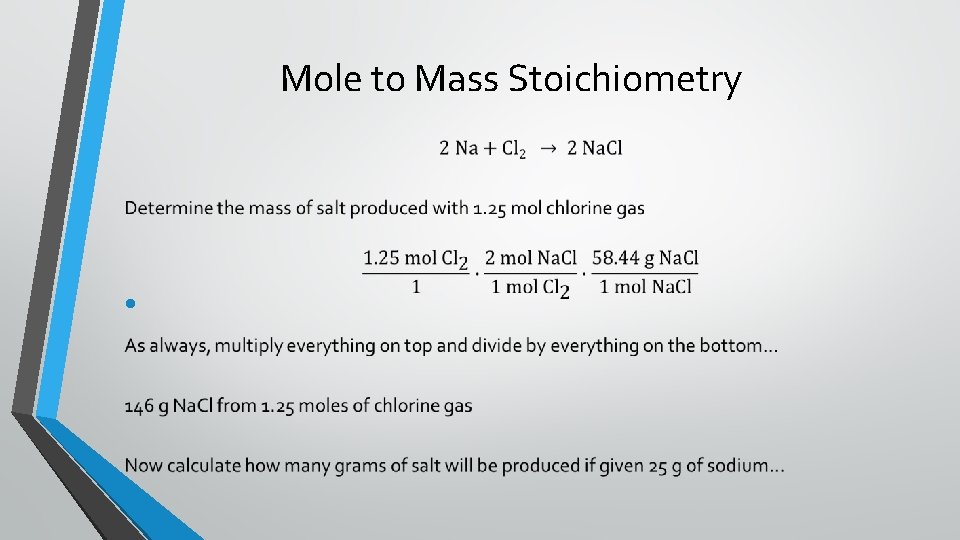
Mole to Mass Stoichiometry •

Solution •

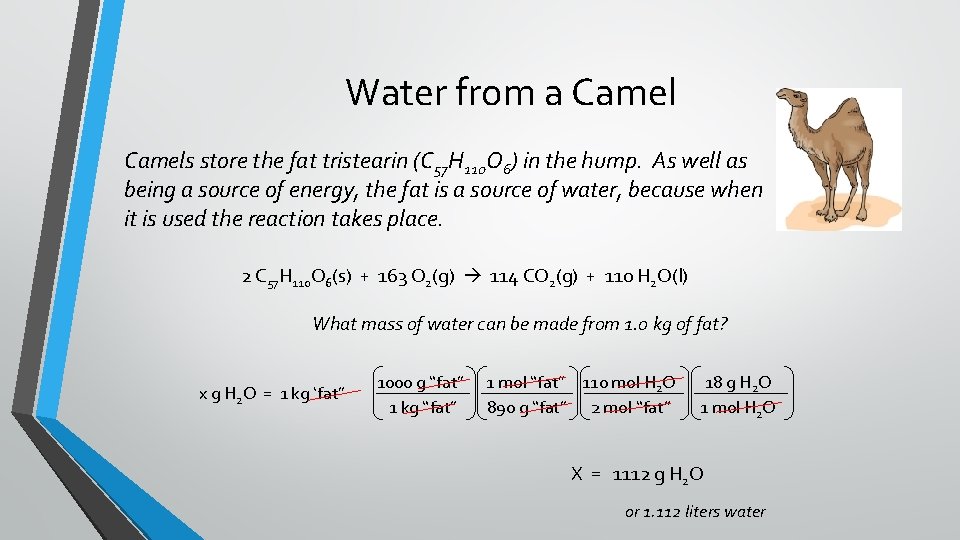
Water from a Camels store the fat tristearin (C 57 H 110 O 6) in the hump. As well as being a source of energy, the fat is a source of water, because when it is used the reaction takes place. 2 C 57 H 110 O 6(s) + 163 O 2(g) 114 CO 2(g) + 110 H 2 O(l) What mass of water can be made from 1. 0 kg of fat? x g H 2 O = 1 kg ‘fat” 1000 g “fat” 1 kg “fat” 1 mol “fat” 110 mol H 2 O 890 g “fat” 2 mol “fat” 18 g H 2 O 1 mol H 2 O X = 1112 g H 2 O or 1. 112 liters water

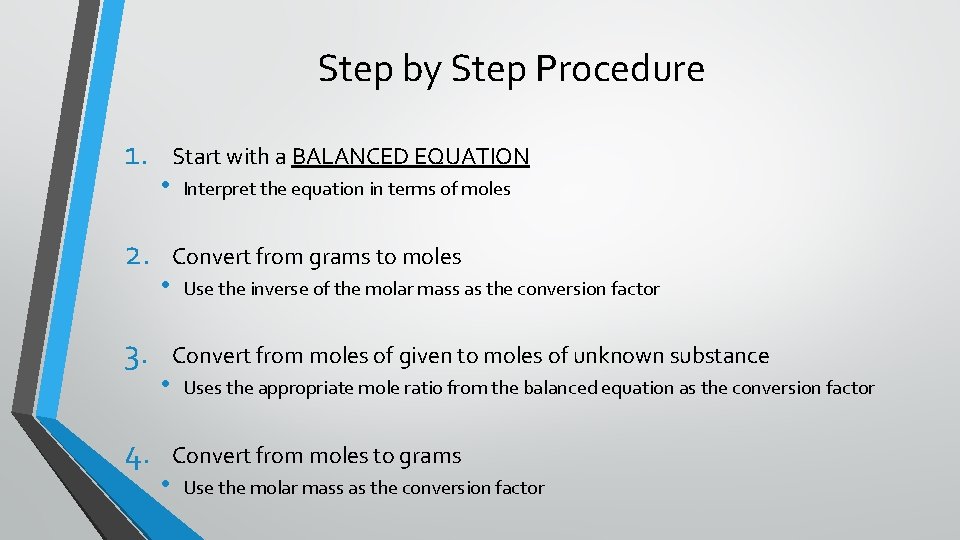
Step by Step Procedure 1. 2. 3. 4. • • Start with a BALANCED EQUATION Interpret the equation in terms of moles Convert from grams to moles Use the inverse of the molar mass as the conversion factor Convert from moles of given to moles of unknown substance Uses the appropriate mole ratio from the balanced equation as the conversion factor Convert from moles to grams Use the molar mass as the conversion factor

Limiting Reactants The reason why chemical reactions stop • Limiting reactant: substance that limits the extent of the reaction and thereby determines the amount of products • Excess Reactants: left-over reactants These will be calculated using stoichiometry

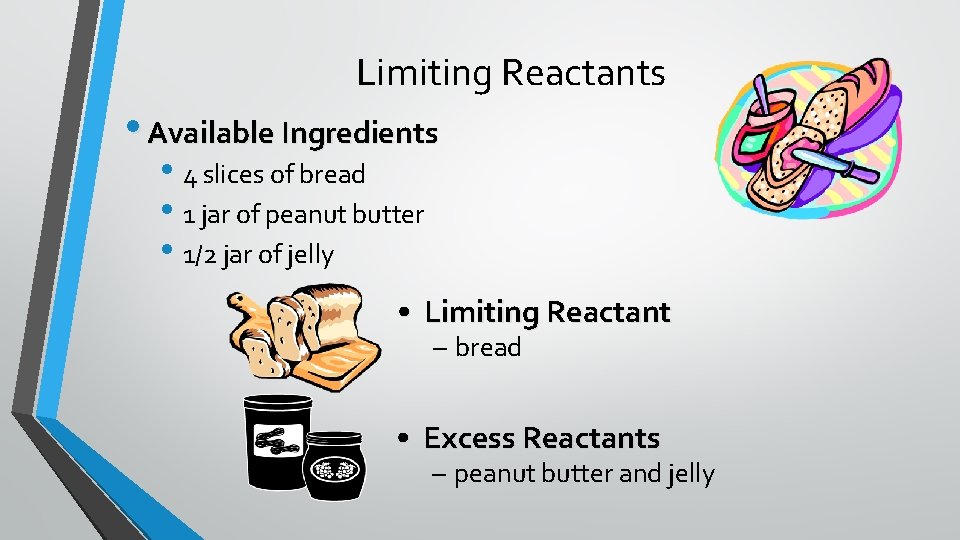
Limiting Reactants • Available Ingredients • 4 slices of bread • 1 jar of peanut butter • 1/2 jar of jelly • Limiting Reactant – bread • Excess Reactants – peanut butter and jelly

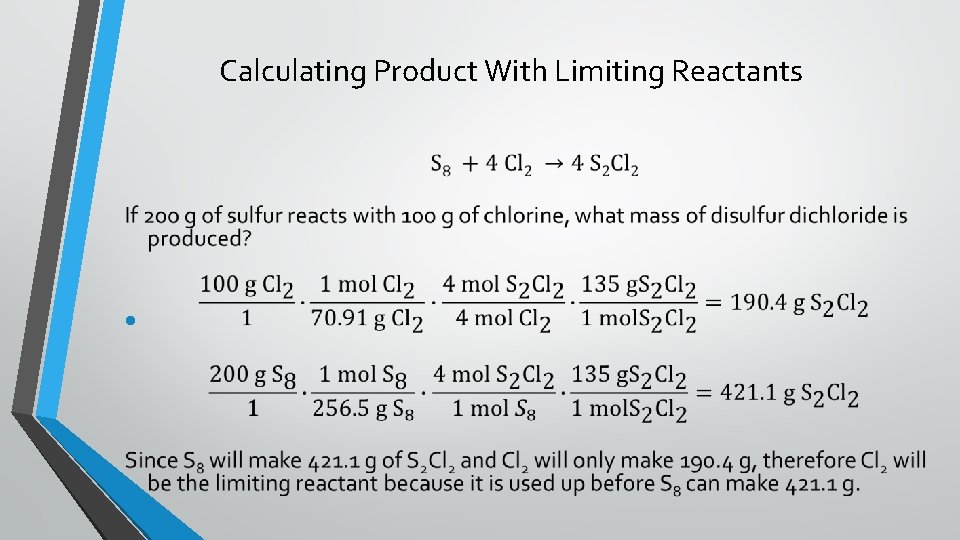
Calculating Product With Limiting Reactants •

Why Use an Excess Reactant? 1. Some reactions will actually stop with low concentrations of reactants so an excess is used to completely use the limiting reactant • When this is done in the laboratory, use the least expensive reactant for the excess reactant 2. It can speed up a chemical reaction • (Remember concentrations!)

Percent Yield •