Unit 8 Stoichiometry Continued Percent Composition Percent composition






- Slides: 15

Unit 8 Stoichiometry… Continued

Percent Composition Percent composition - the percent by mass of each element in a compound Percent composition = part x 100 whole Just like calculating your grade on an assignment

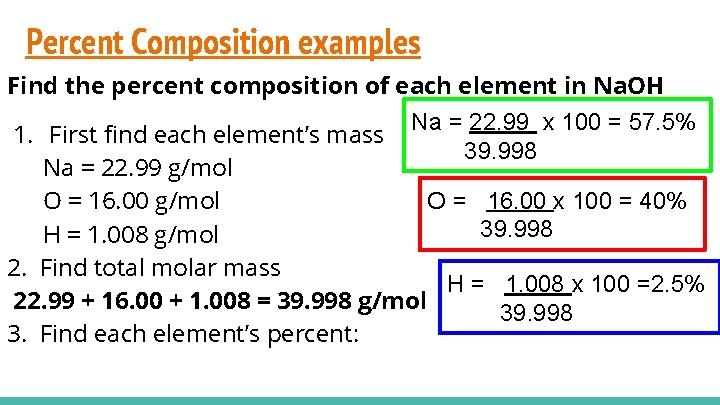
Percent Composition examples Find the percent composition of each element in Na. OH Na = 22. 99 x 100 = 57. 5% 1. First find each element’s mass 39. 998 Na = 22. 99 g/mol O = 16. 00 x 100 = 40% O = 16. 00 g/mol 39. 998 H = 1. 008 g/mol 2. Find total molar mass H = 1. 008 x 100 =2. 5% 22. 99 + 16. 00 + 1. 008 = 39. 998 g/mol 39. 998 3. Find each element’s percent:

Percent Composition Practice Find the percent composition of each element in the following molecules: Al 2(SO 4)3 Ca 3(PO 4)2

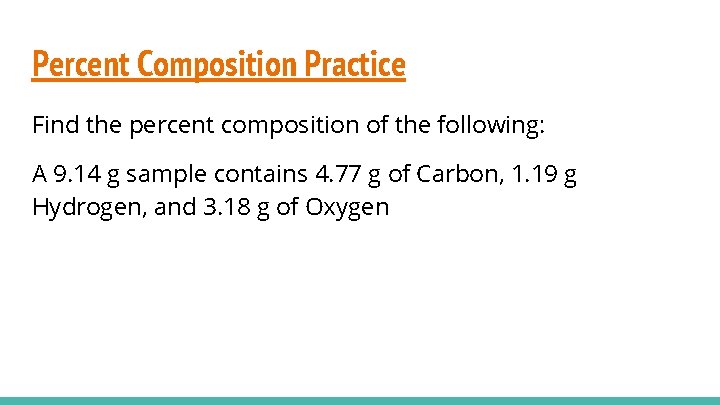
Percent Composition Practice Find the percent composition of the following: A 9. 14 g sample contains 4. 77 g of Carbon, 1. 19 g Hydrogen, and 3. 18 g of Oxygen

Hydrates A Hydrate is a compound that contains water in its molecular formula. Ex. This is copper sulfate pentahydrate. (Notice the prefix is similar to those used in ionic bonds)

Hydrates When heat is added to a hydrate, water is released as a vapor and an anhydrous solid remains. → Cu. SO 4 + 5 H 2 O Many substances exist as hydrates. (Ex. Popcorn, Borax, Gypsum, Epsom Salt) Using the following reaction: B 2 H 6 + 3 O 2 → 2 HBO 2 + 2 H 2 O a. What mass of oxygen will be needed to burn 36. 1 g of B 2 H 6? b. How many molecules of water are produced from 1. 2 g of B 2 H 6?

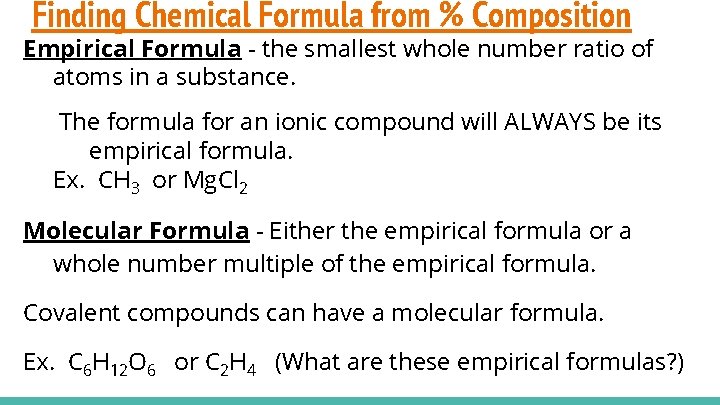
Finding Chemical Formula from % Composition Empirical Formula - the smallest whole number ratio of atoms in a substance. The formula for an ionic compound will ALWAYS be its empirical formula. Ex. CH 3 or Mg. Cl 2 Molecular Formula - Either the empirical formula or a whole number multiple of the empirical formula. Covalent compounds can have a molecular formula. Ex. C 6 H 12 O 6 or C 2 H 4 (What are these empirical formulas? )

Empirical Formula To calculate empirical formula: 1. Find the mass or % mass of each element. 2. Find the number of moles of each element. 3. Divide the number of moles by the smallest number found. 4. If needed, multiply by 2, 3, or 4 get obtain whole numbers. Tip: if you get a fraction, look for the following to know what to multiply by: 0. 5 multiply by 2 0. 33 multiply by 3 0. 25 multiply by 4

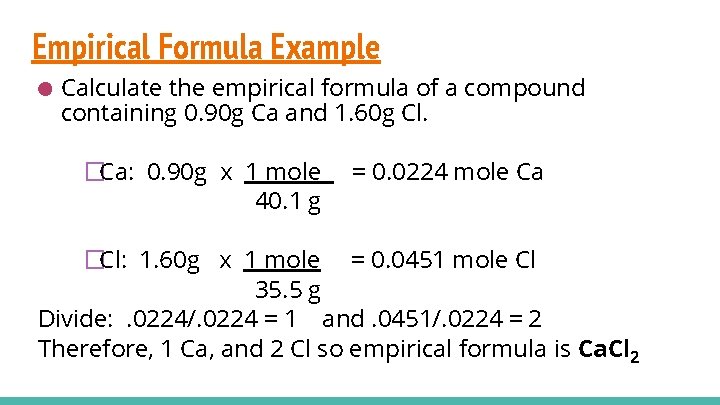
Empirical Formula Example ● Calculate the empirical formula of a compound containing 0. 90 g Ca and 1. 60 g Cl. �Ca: 0. 90 g x 1 mole 40. 1 g = 0. 0224 mole Ca �Cl: 1. 60 g x 1 mole = 0. 0451 mole Cl 35. 5 g Divide: . 0224/. 0224 = 1 and. 0451/. 0224 = 2 Therefore, 1 Ca, and 2 Cl so empirical formula is Ca. Cl 2

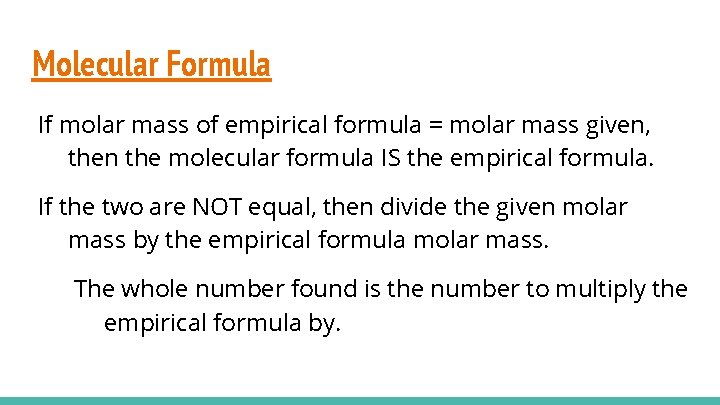
Molecular Formula If molar mass of empirical formula = molar mass given, then the molecular formula IS the empirical formula. If the two are NOT equal, then divide the given molar mass by the empirical formula molar mass. The whole number found is the number to multiply the empirical formula by.

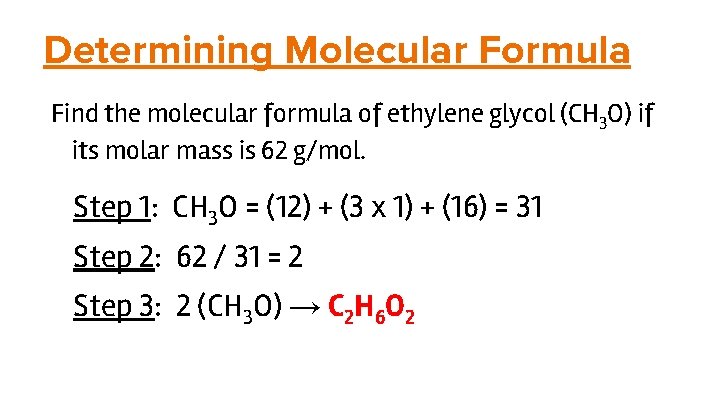
Determining Molecular Formula Find the molecular formula of ethylene glycol (CH 3 O) if its molar mass is 62 g/mol. Step 1: CH 3 O = (12) + (3 x 1) + (16) = 31 Step 2: 62 / 31 = 2 Step 3: 2 (CH 3 O) → C 2 H 6 O 2

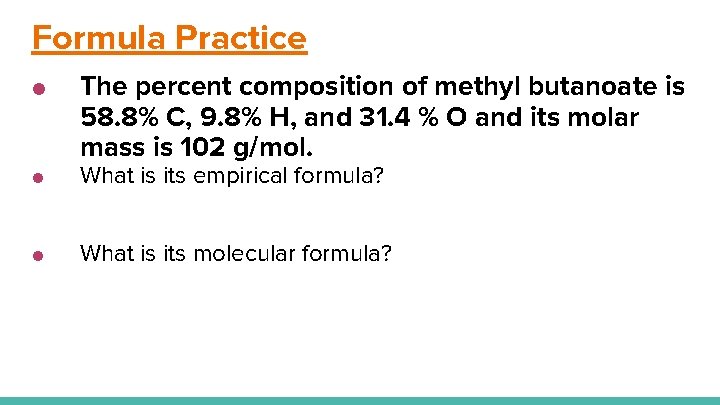
Formula Practice ● The percent composition of methyl butanoate is 58. 8% C, 9. 8% H, and 31. 4 % O and its molar mass is 102 g/mol. ● What is its empirical formula? ● What is its molecular formula?

Empirical Formula Practice A compound was analyzed and found to contain 13. 5 g Ca, 10. 8 g O, and 0. 675 g H. What is the empirical formula of the compound?

Empirical Formula and Molecular Formula Practice An unknown compound is found to contain 40. 0% carbon, 6. 7% hydrogen and 53. 3% oxygen with a molecular mass of 60. 0 g/mol. What is the molecular formula of the unknown compound?