States of Matter and Phase Changes Its just




















- Slides: 20

States of Matter and Phase Changes “It’s just a phase”

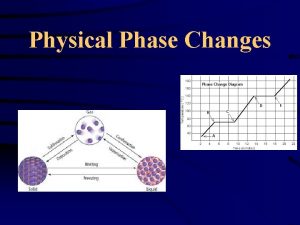
States of Matter l. Solid, liquid and gas (plasma and BEC). l. Changes between states are called “phase changes”. l. Caused by a change of heat or pressure. More often heat.

Solids l. Molecules are tightly packed together. l. Particles vibrate in place. l. Definite shape and volume. l. Usually higher densities.



Liquids l. Particles are not so tightly packed. l. Molecules move slowly and slide past each other. l. Medium density. l. Definite volume, but not a definite shape.


Gases l Particles spread out as the container will allow. l Particles are moving very quickly (1000 km/sec). l Low density. l No definite shape or volume.


Phase Changes l. When a substance changes states.


Melting l. Transformation of a solid to a liquid. l. Adding of thermal energy. l. Ice becoming liquid water.

Freezing l. Transformation of a liquid to a solid. l. Removing thermal energy. l. Liquid water becoming ice.

Boiling/Evaporation l. Transformation of a liquid to a gas. l. Adding of thermal energy. l. Liquid water to water vapor (steam).

Condensation l. Transformation of a gas to a liquid. l. Removing thermal energy. l. Water vapor becoming liquid water.

Sublimation l. Transformation of a substance to a gas from a solid state with no liquid transition. l. Adding thermal energy quickly. l. Dry Ice does this.

Deposition l. When a gas transforms into a solid without transitioning through a liquid state. l. Removing thermal energy quickly. l. Ex. Frost forming on windows.

• During a phase change the temperature does not change, but the amount of energy does.


Where does all the energy go? l During a phase change energy is added, but the temperature does not increase. l The energy goes toward breaking up forces between the particles.
 Definition of substance
Definition of substance Stp law
Stp law 6 common phase changes
6 common phase changes Changes in latitudes, changes in attitudes meaning
Changes in latitudes, changes in attitudes meaning Chemical vs physical change
Chemical vs physical change Properties and changes of matter worksheet
Properties and changes of matter worksheet Matter-properties and changes answer key
Matter-properties and changes answer key Which is a “big idea” for matter and change?
Which is a “big idea” for matter and change? Eating food physical or chemical change
Eating food physical or chemical change Who is present when juliet awakens
Who is present when juliet awakens Mobile phase and stationary phase
Mobile phase and stationary phase Mobile phase
Mobile phase Which detector used in hplc
Which detector used in hplc Four phase changes
Four phase changes Solid to liquid endothermic or exothermic
Solid to liquid endothermic or exothermic 6 phase changes
6 phase changes Phase changes worksheet answer key
Phase changes worksheet answer key Are phase changes reversible
Are phase changes reversible Which of these phase changes is an endothermic process?
Which of these phase changes is an endothermic process? Phase changes
Phase changes Chemical properties
Chemical properties