Spring 2007 Chap 10 Chaingrowth Polymerization ChainGrowth Polymerization
![Kinetic Chain Reaction Spring 2007 [M ] elimination methods Steady-State Assumption Radical concentration increases Kinetic Chain Reaction Spring 2007 [M ] elimination methods Steady-State Assumption Radical concentration increases](https://slidetodoc.com/presentation_image_h2/718fbf0693b929d0e5cea928d7712f6e/image-29.jpg)
![Spring 2007 Kinetic Chain Reaction Mostly in case of f<1 system → [I 2]1/2 Spring 2007 Kinetic Chain Reaction Mostly in case of f<1 system → [I 2]1/2](https://slidetodoc.com/presentation_image_h2/718fbf0693b929d0e5cea928d7712f6e/image-30.jpg)
![Spring 2007 Ceiling Temperature Polymer-Depolymerization Equilibria k sec-1 kdp kp[M]- kdp Tc : No Spring 2007 Ceiling Temperature Polymer-Depolymerization Equilibria k sec-1 kdp kp[M]- kdp Tc : No](https://slidetodoc.com/presentation_image_h2/718fbf0693b929d0e5cea928d7712f6e/image-45.jpg)
- Slides: 48

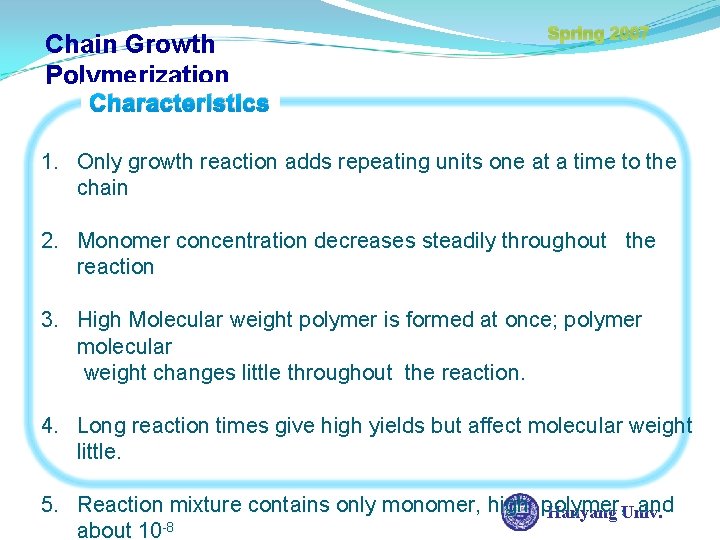
Spring 2007 Chap 10. Chain-growth Polymerization Chain-Growth Polymerization (Addition) Processes 1. Free radical Initiation Processes 2. Cationically Initiated Processes 3. Anionically Initiated Processes 4. Group Transfer Polymerization 5. Coordination Polymerization Hanyang Univ.

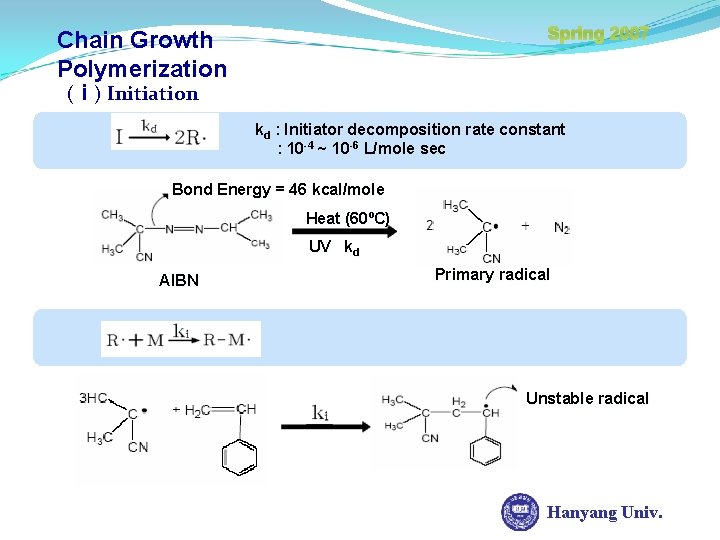
Chain Growth Polymerization Spring 2007 Characteristics 1. Only growth reaction adds repeating units one at a time to the chain 2. Monomer concentration decreases steadily throughout the reaction 3. High Molecular weight polymer is formed at once; polymer molecular weight changes little throughout the reaction. 4. Long reaction times give high yields but affect molecular weight little. 5. Reaction mixture contains only monomer, high polymer, and Hanyang Univ. about 10 -8

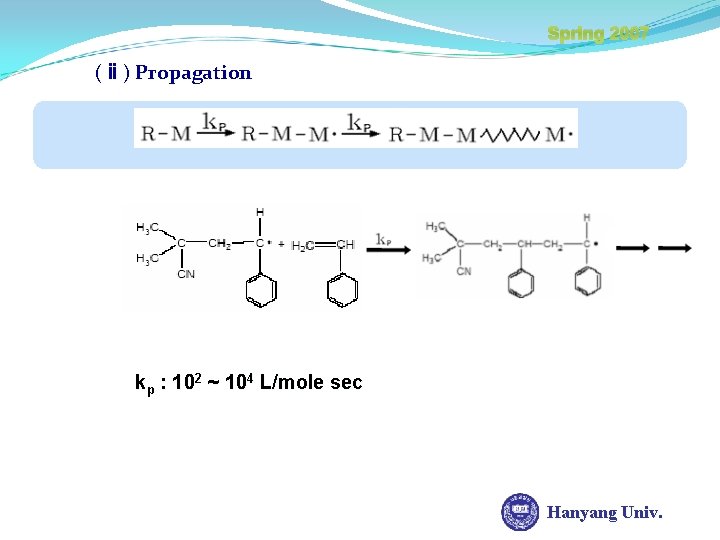
Spring 2007 Chain Growth Polymerization (ⅰ) Initiation kd : Initiator decomposition rate constant I : 10 -4 ~ 10 -6 L/mole sec Bond Energy = 46 kcal/mole Heat (60ºC) UV kd AIBN Primary radical Unstable radical Hanyang Univ.

Spring 2007 (ⅱ) Propagation kp : 102 ~ 104 L/mole sec Hanyang Univ.

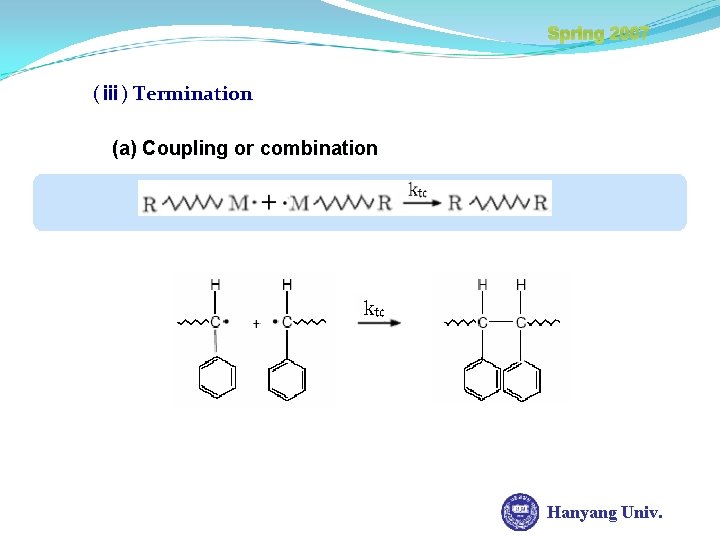
Spring 2007 (ⅲ) Termination (a) Coupling or combination Hanyang Univ.

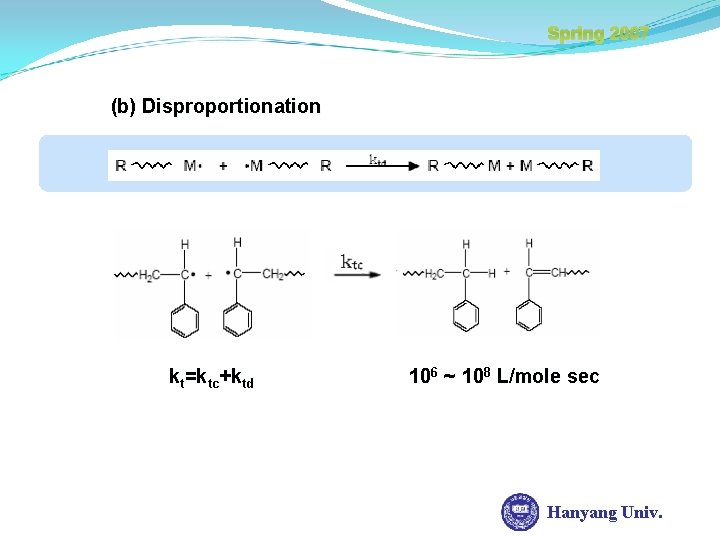
Spring 2007 (b) Disproportionation kt=ktc+ktd 106 ~ 108 L/mole sec Hanyang Univ.

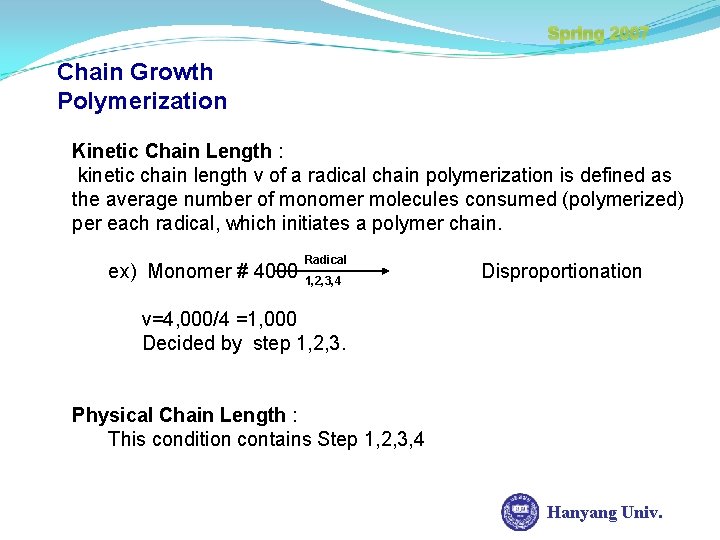
Spring 2007 Chain Growth Polymerization Kinetic Chain Length : kinetic chain length v of a radical chain polymerization is defined as the average number of monomer molecules consumed (polymerized) per each radical, which initiates a polymer chain. ex) Monomer # 4000 Radical 1, 2, 3, 4 Disproportionation ν=4, 000/4 =1, 000 Decided by step 1, 2, 3. Physical Chain Length : This condition contains Step 1, 2, 3, 4 Hanyang Univ.

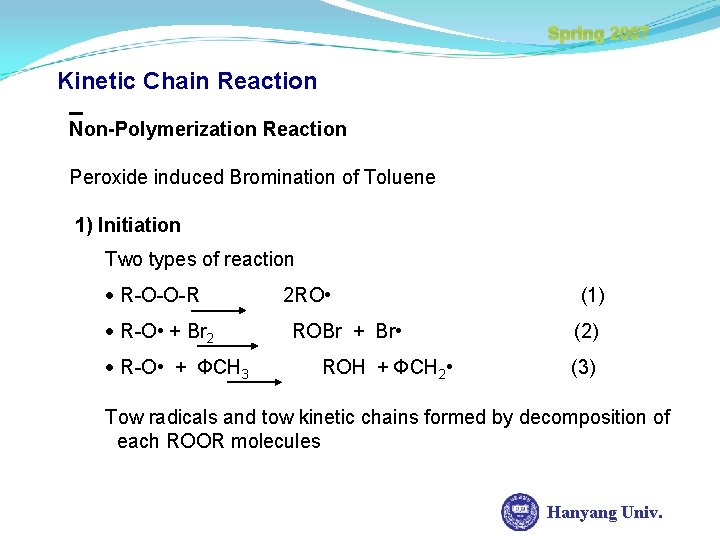
Spring 2007 Kinetic Chain Reaction Non-Polymerization Reaction Peroxide induced Bromination of Toluene 1) Initiation Two types of reaction R-O-O-R R-O • + Br 2 R-O • + ФCH 3 2 RO • ROBr + Br • ROH + ФCH 2 • (1) (2) (3) Tow radicals and tow kinetic chains formed by decomposition of each ROOR molecules Hanyang Univ.

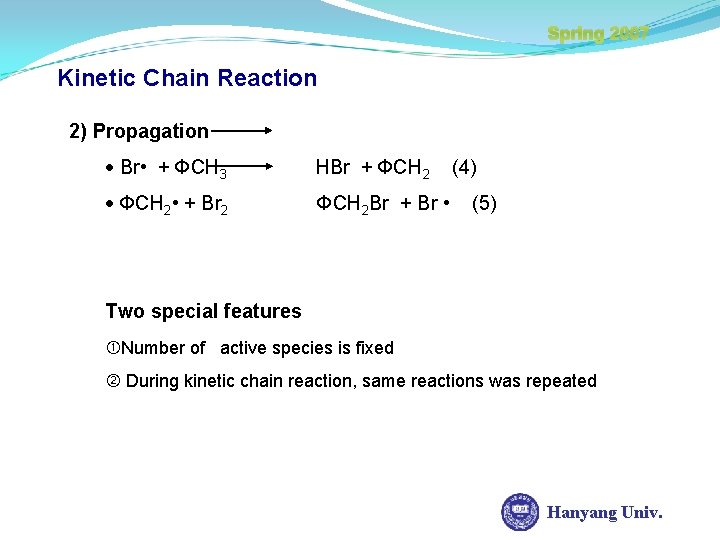
Spring 2007 Kinetic Chain Reaction 2) Propagation Br • + ФCH 3 HBr + ФCH 2 • + Br 2 ФCH 2 Br + Br • (4) (5) Two special features Number of active species is fixed During kinetic chain reaction, same reactions was repeated Hanyang Univ.

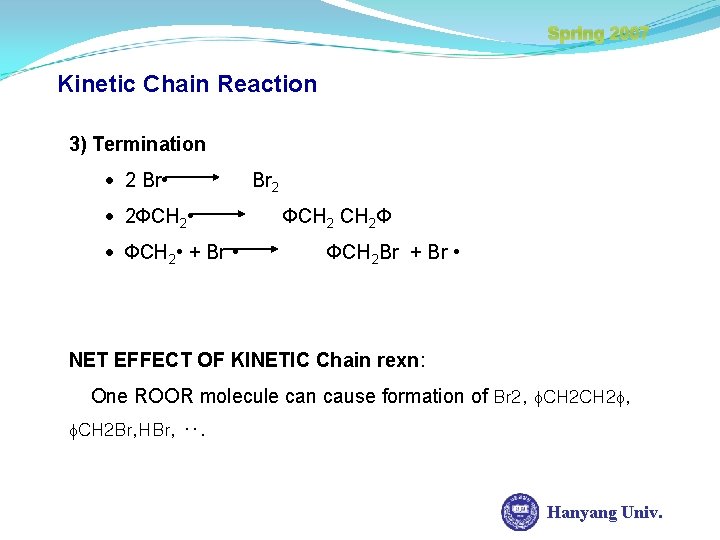
Spring 2007 Kinetic Chain Reaction 3) Termination 2 Br • 2 ФCH 2 • + Br • Br 2 ФCH 2 Ф ФCH 2 Br + Br • NET EFFECT OF KINETIC Chain rexn: One ROOR molecule can cause formation of Br 2, CH 2 Br, HBr, ‥. Hanyang Univ.

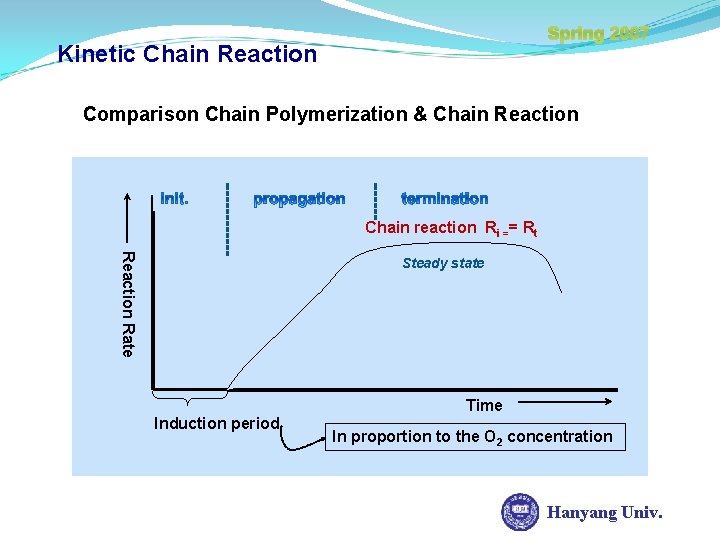
Spring 2007 Kinetic Chain Reaction Comparison Chain Polymerization & Chain Reaction Chain reaction Ri == Rt Reaction Rate Steady state Time Induction period In proportion to the O 2 concentration Hanyang Univ.

Spring 2007 Kinetic Chain Reaction In case of Chain reaction, there are mainly induction periods, due to the inhibitor. If an active center is formed, the reaction rate accelerate and then come to steady state. The whole reaction rate is reaching plateau region. Linear Chain-Growth: Polymer of high DPn found easily in early reaction Linear Step-Growth: high extent of reaction value required to obtain high DPn Hanyang Univ.

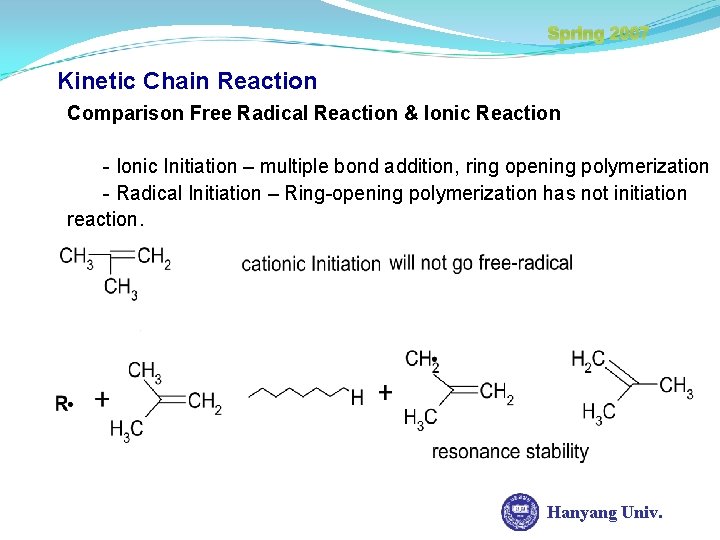
Spring 2007 Kinetic Chain Reaction Comparison Free Radical Reaction & Ionic Reaction - Ionic Initiation – multiple bond addition, ring opening polymerization - Radical Initiation – Ring-opening polymerization has not initiation reaction. Hanyang Univ.

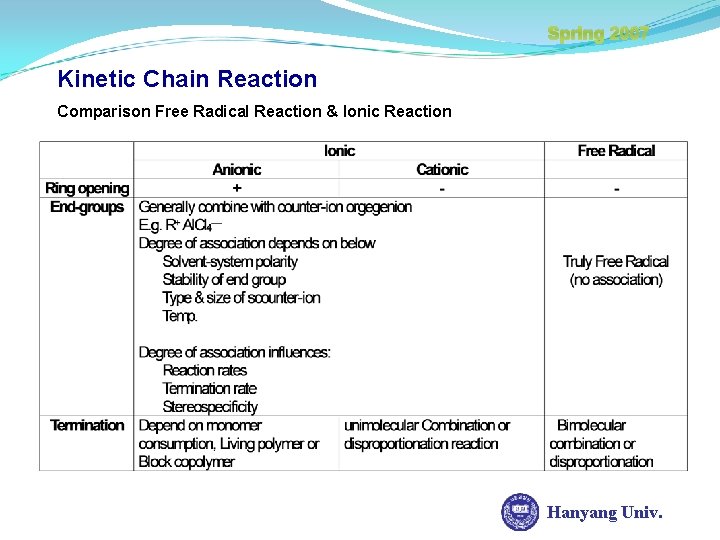
Spring 2007 Kinetic Chain Reaction Comparison Free Radical Reaction & Ionic Reaction Hanyang Univ.

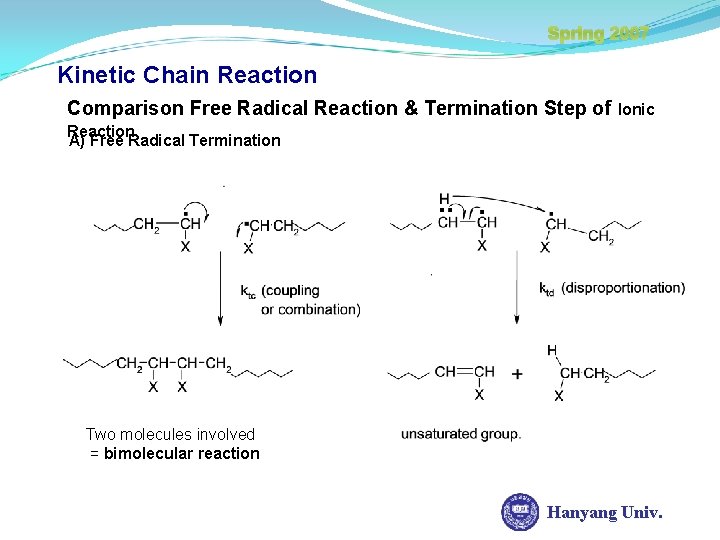
Spring 2007 Kinetic Chain Reaction Comparison Free Radical Reaction & Termination Step of Ionic Reaction A) Free Radical Termination Two molecules involved = bimolecular reaction Hanyang Univ.

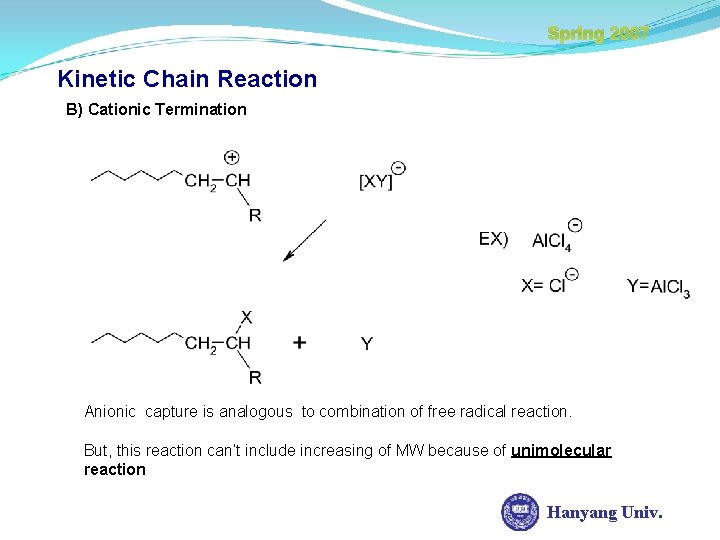
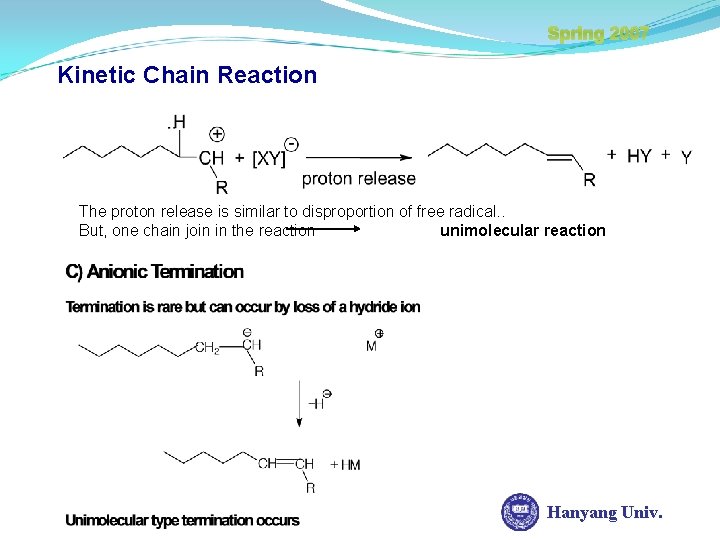
Spring 2007 Kinetic Chain Reaction B) Cationic Termination Anionic capture is analogous to combination of free radical reaction. But, this reaction can’t include increasing of MW because of unimolecular reaction Hanyang Univ.

Spring 2007 Kinetic Chain Reaction The proton release is similar to disproportion of free radical. . But, one chain join in the reaction unimolecular reaction Hanyang Univ.

Spring 2007 Kinetic Chain Reaction Hanyang Univ.

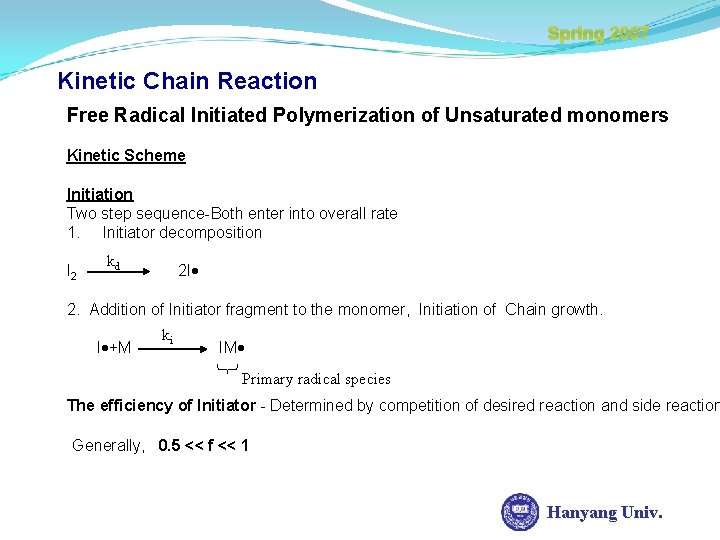
Spring 2007 Kinetic Chain Reaction Free Radical Initiated Polymerization of Unsaturated monomers Kinetic Scheme Initiation Two step sequence-Both enter into overall rate 1. Initiator decomposition I 2 kd 2 I 2. Addition of Initiator fragment to the monomer, Initiation of Chain growth. I +M ki IM Primary radical species The efficiency of Initiator - Determined by competition of desired reaction and side reaction Generally, 0. 5 << f << 1 Hanyang Univ.

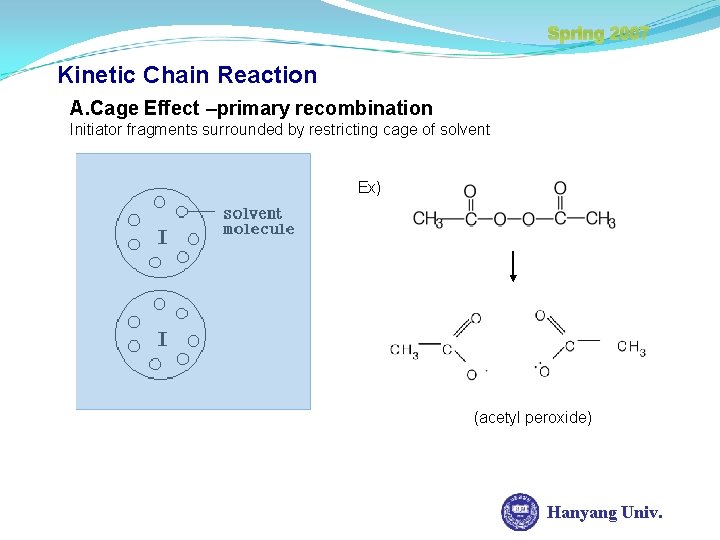
Spring 2007 Kinetic Chain Reaction A. Cage Effect –primary recombination Initiator fragments surrounded by restricting cage of solvent Ex) (acetyl peroxide) Hanyang Univ.

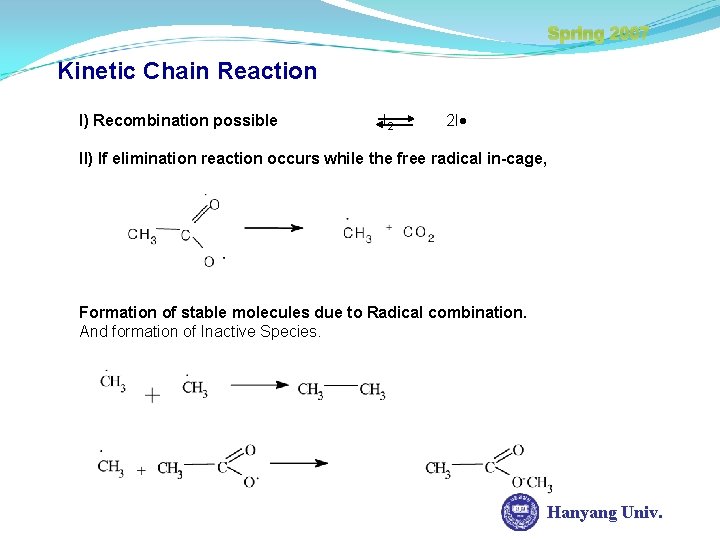
Spring 2007 Kinetic Chain Reaction I) Recombination possible I 2 2 I II) If elimination reaction occurs while the free radical in-cage, Formation of stable molecules due to Radical combination. And formation of Inactive Species. Hanyang Univ.

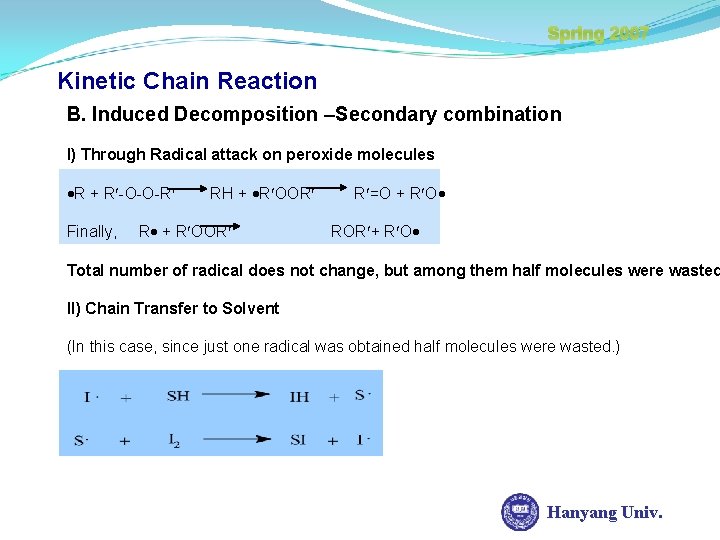
Spring 2007 Kinetic Chain Reaction B. Induced Decomposition –Secondary combination I) Through Radical attack on peroxide molecules R + R -O-O-R Finally, RH + R OOR R =O + R O ROR + R O Total number of radical does not change, but among them half molecules were wasted II) Chain Transfer to Solvent (In this case, since just one radical was obtained half molecules were wasted. ) Hanyang Univ.

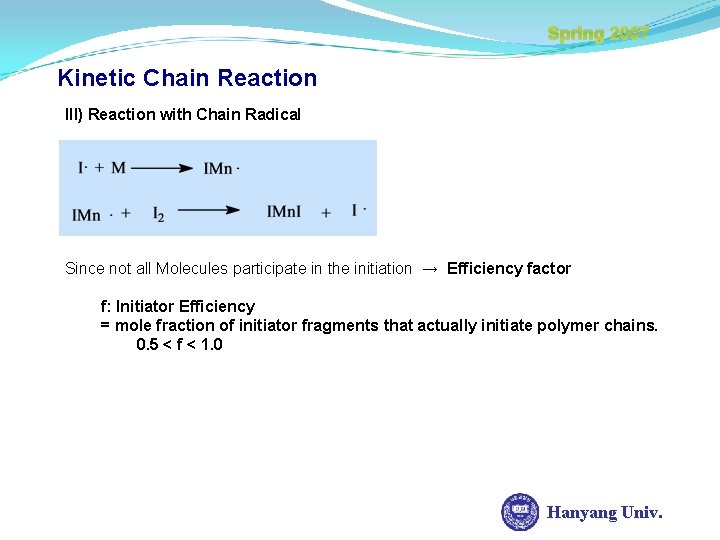
Spring 2007 Kinetic Chain Reaction III) Reaction with Chain Radical Since not all Molecules participate in the initiation → Efficiency factor f: Initiator Efficiency = mole fraction of initiator fragments that actually initiate polymer chains. 0. 5 < f < 1. 0 Hanyang Univ.

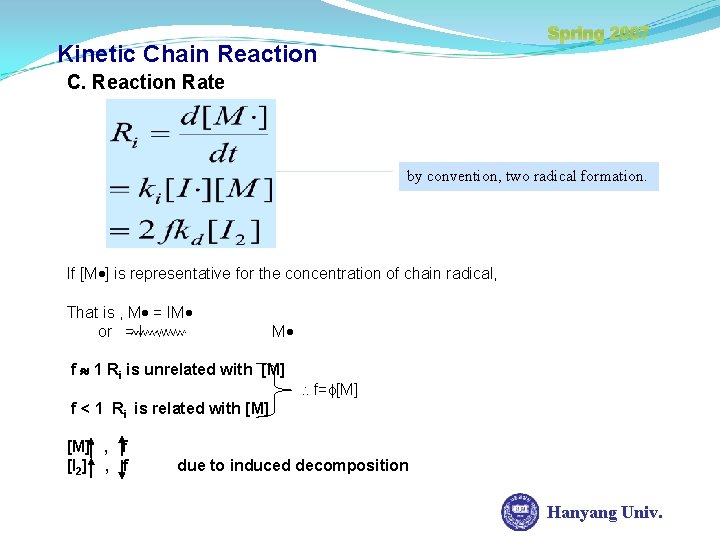
Spring 2007 Kinetic Chain Reaction C. Reaction Rate by convention, two radical formation. If [M ] is representative for the concentration of chain radical, That is , M = IM or = I M f 1 Ri is unrelated with [M] f= [M] f < 1 Ri is related with [M] , f [I 2] , f due to induced decomposition Hanyang Univ.

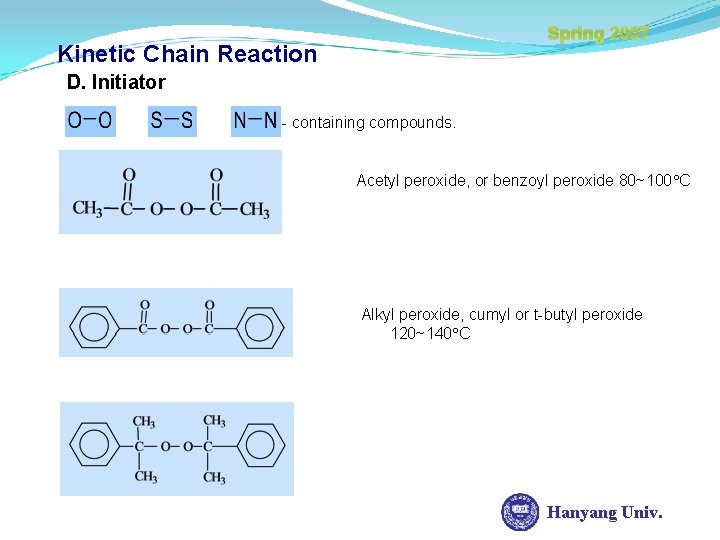
Spring 2007 Kinetic Chain Reaction D. Initiator - containing compounds. Acetyl peroxide, or benzoyl peroxide 80~100 C Alkyl peroxide, cumyl or t-butyl peroxide 120~140 C Hanyang Univ.

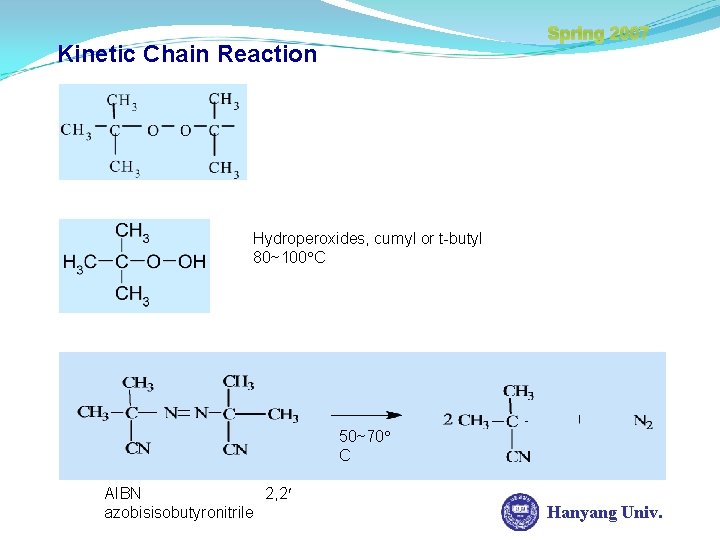
Spring 2007 Kinetic Chain Reaction Hydroperoxides, cumyl or t-butyl 80~100 C 50~70 C AIBN 2, 2 azobisisobutyronitrile Hanyang Univ.

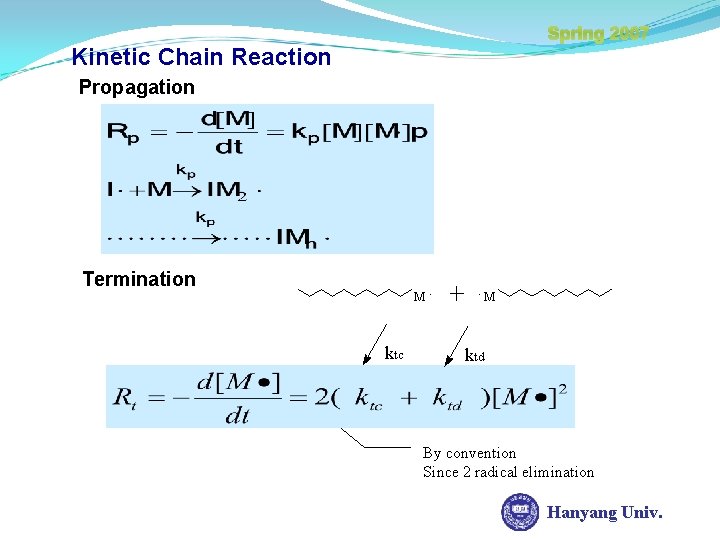
Spring 2007 Kinetic Chain Reaction Propagation Termination M. ktc . M ktd By convention Since 2 radical elimination Hanyang Univ.

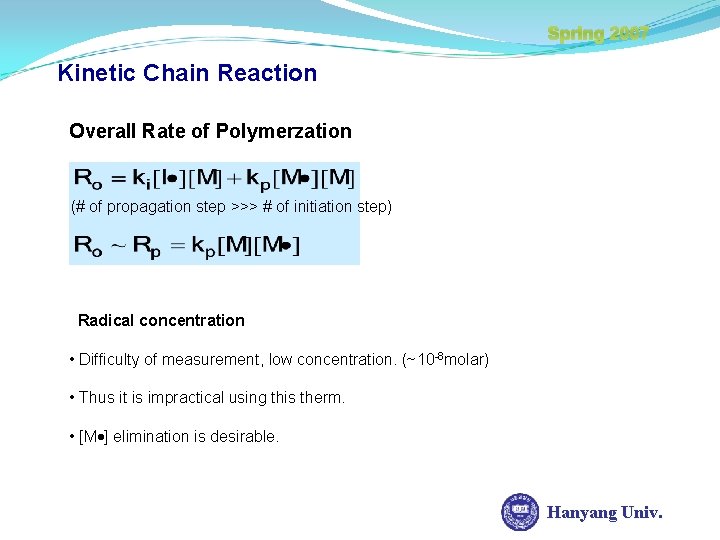
Spring 2007 Kinetic Chain Reaction Overall Rate of Polymerzation (# of propagation step >>> # of initiation step) Radical concentration • Difficulty of measurement, low concentration. (~10 -8 molar) • Thus it is impractical using this therm. • [M ] elimination is desirable. Hanyang Univ.
![Kinetic Chain Reaction Spring 2007 M elimination methods SteadyState Assumption Radical concentration increases Kinetic Chain Reaction Spring 2007 [M ] elimination methods Steady-State Assumption Radical concentration increases](https://slidetodoc.com/presentation_image_h2/718fbf0693b929d0e5cea928d7712f6e/image-29.jpg)
Kinetic Chain Reaction Spring 2007 [M ] elimination methods Steady-State Assumption Radical concentration increases at the start, comes to steady state simutaneously. and then reaction rate change becomes 0. (active centers created and destroyed at the sam Ri = R t Hanyang Univ.
![Spring 2007 Kinetic Chain Reaction Mostly in case of f1 system I 212 Spring 2007 Kinetic Chain Reaction Mostly in case of f<1 system → [I 2]1/2](https://slidetodoc.com/presentation_image_h2/718fbf0693b929d0e5cea928d7712f6e/image-30.jpg)
Spring 2007 Kinetic Chain Reaction Mostly in case of f<1 system → [I 2]1/2 (Square Root Dependence of [I 2]) MMA using BPO ※ Odian Fig. 3 -4 Rp Vinyl Acetate using AIBN [I 2]1/2 2 BPO -CO 2 300 C + N 2 Azobisisobutyronitrile Hanyang Univ.

Kinetic Chain Reaction Spring 2007 In case f < 1, but SRD is not applicable, Because f is ‘dependent’ on [M] Why? Due to induced decomposition of toluene + [I 2] Hanyang Univ.

Kinetic Chain Length (KCL) Spring 2007 At S-S assumption (1) Disproportionation Knowing that (2) Coupling or combination Hanyang Univ.

Kinetic Chain Length (KCL) Spring 2007 (3) Both (1)+(2) Hanyang Univ.

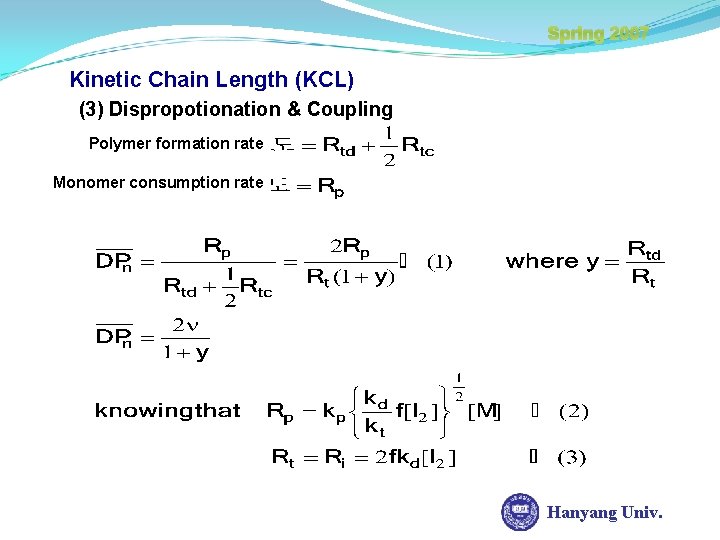
Kinetic Chain Length (KCL) Spring 2007 Degree of Polymerization The more concentration of monomer, DP The less concentration of initiator, DP Monomer consumption rate polymer formation rate (1) Dispropotionation (2) Coupling Hanyang Univ.

Spring 2007 Kinetic Chain Length (KCL) (3) Dispropotionation & Coupling Polymer formation rate Monomer consumption rate Hanyang Univ.

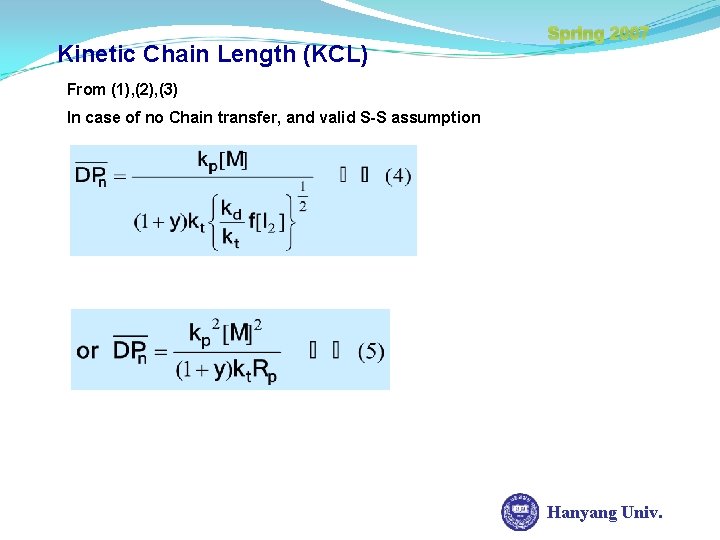
Kinetic Chain Length (KCL) Spring 2007 From (1), (2), (3) In case of no Chain transfer, and valid S-S assumption Hanyang Univ.

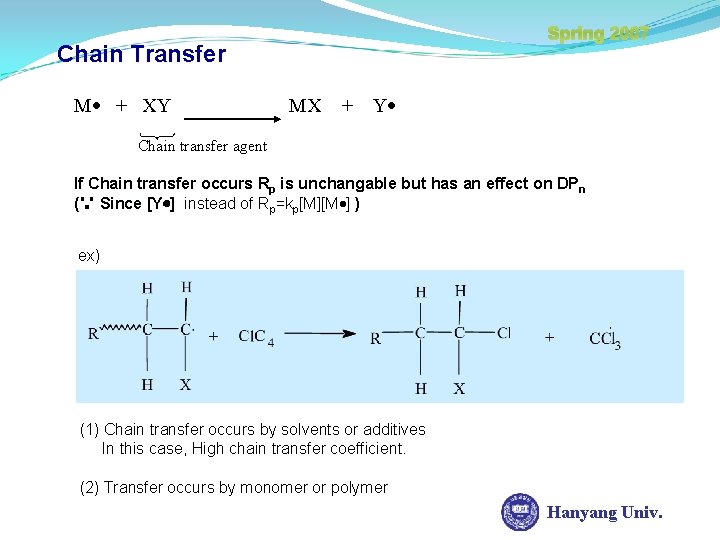
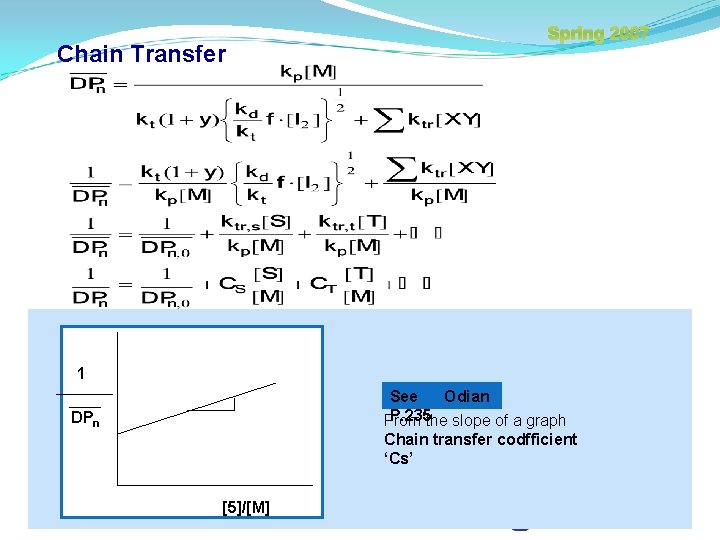
Spring 2007 Chain Transfer M + XY MX + Y Chain transfer agent If Chain transfer occurs Rp is unchangable but has an effect on DPn (∵ Since [Y ] instead of Rp=kp[M][M ] ) ex) (1) Chain transfer occurs by solvents or additives In this case, High chain transfer coefficient. (2) Transfer occurs by monomer or polymer Hanyang Univ.

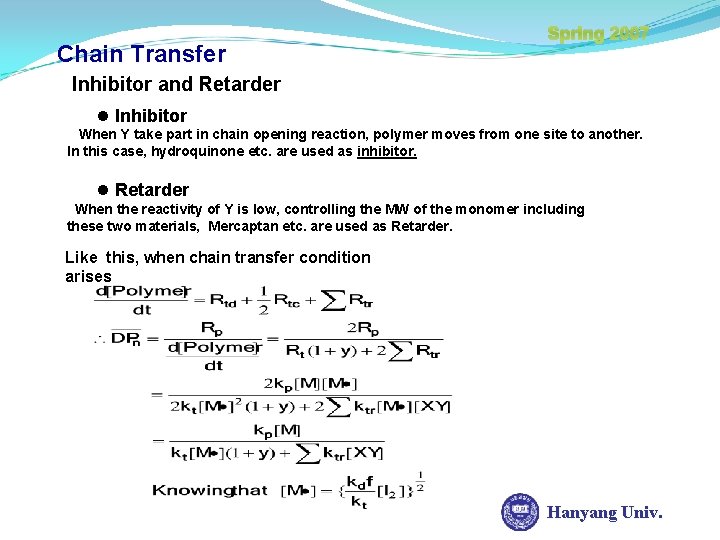
Chain Transfer Spring 2007 Inhibitor and Retarder l Inhibitor When Y take part in chain opening reaction, polymer moves from one site to another. In this case, hydroquinone etc. are used as inhibitor. l Retarder When the reactivity of Y is low, controlling the MW of the monomer including these two materials, Mercaptan etc. are used as Retarder. Like this, when chain transfer condition arises Hanyang Univ.

Chain Transfer Spring 2007 1 See Odian P. 235 the slope of a graph From DPn Chain transfer codfficient ‘Cs’ [5]/[M] Hanyang Univ.

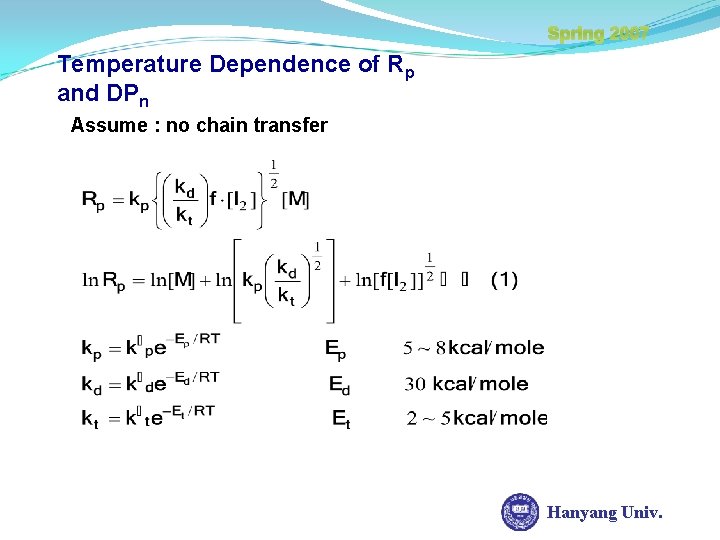
Spring 2007 Temperature Dependence of Rp and DPn Assume : no chain transfer Hanyang Univ.

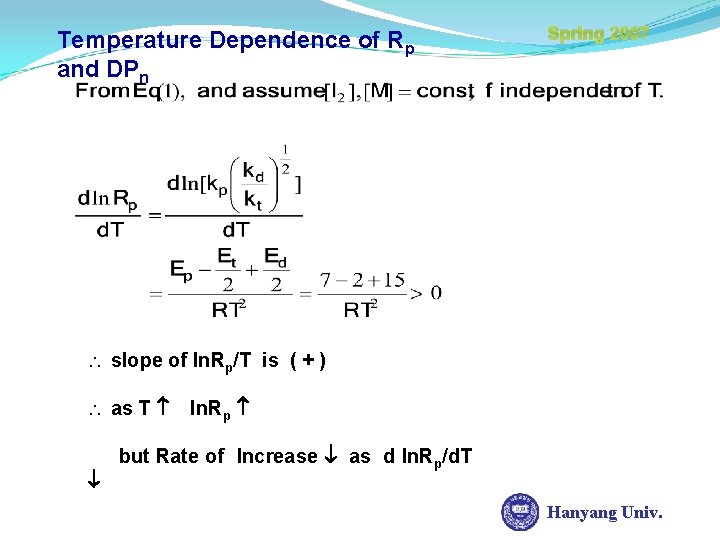
Temperature Dependence of Rp and DPn Spring 2007 slope of ln. Rp/T is ( + ) as T ln. Rp but Rate of Increase as d ln. Rp/d. T Hanyang Univ.

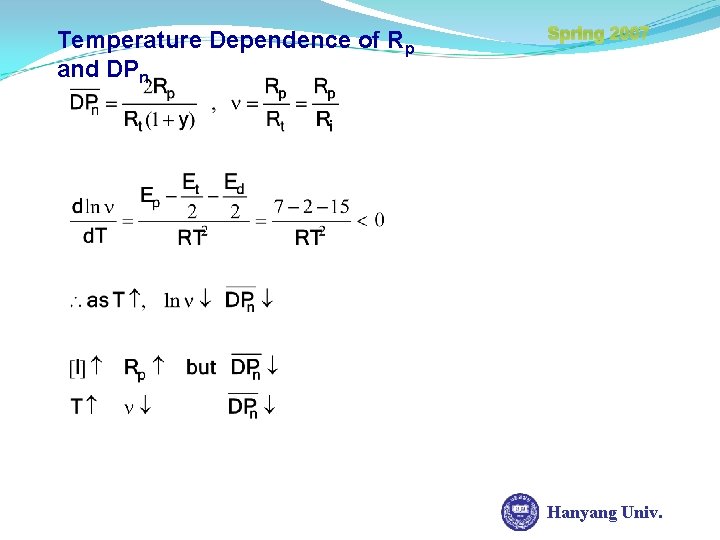
Temperature Dependence of Rp and DPn Spring 2007 Hanyang Univ.

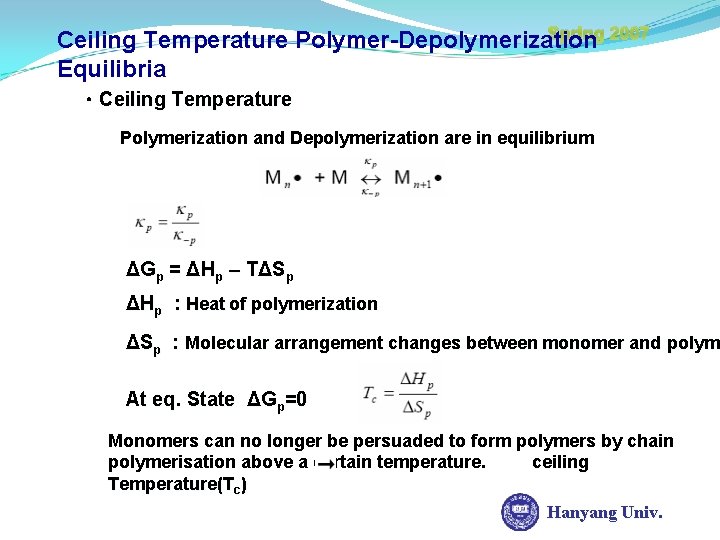
Spring 2007 Ceiling Temperature Polymer-Depolymerization Equilibria ㆍCeiling Temperature Polymerization and Depolymerization are in equilibrium ΔGp = ΔHp – TΔSp ΔHp : Heat of polymerization ΔSp : Molecular arrangement changes between monomer and polyme polym At eq. State ΔGp=0 Monomers can no longer be persuaded to form polymers by chain polymerisation above a certain temperature. ceiling Temperature(Tc) Hanyang Univ.

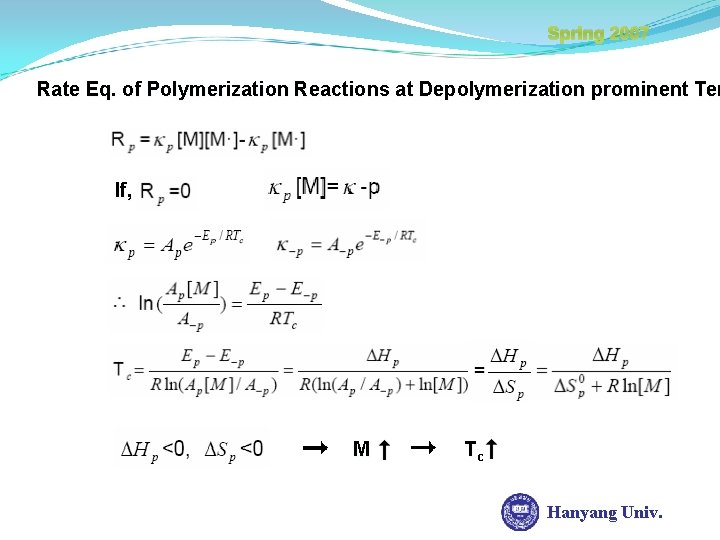
Spring 2007 Rate Eq. of Polymerization Reactions at Depolymerization prominent Tem If, M Tc Hanyang Univ.
![Spring 2007 Ceiling Temperature PolymerDepolymerization Equilibria k sec1 kdp kpM kdp Tc No Spring 2007 Ceiling Temperature Polymer-Depolymerization Equilibria k sec-1 kdp kp[M]- kdp Tc : No](https://slidetodoc.com/presentation_image_h2/718fbf0693b929d0e5cea928d7712f6e/image-45.jpg)
Spring 2007 Ceiling Temperature Polymer-Depolymerization Equilibria k sec-1 kdp kp[M]- kdp Tc : No reaction above Tc 300 400 500 Tc Stable blow Tc Hanyang Univ.

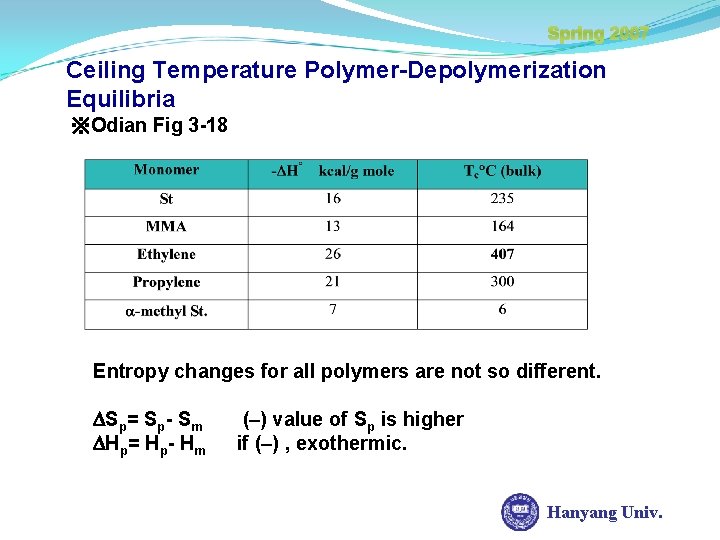
Spring 2007 Ceiling Temperature Polymer-Depolymerization Equilibria ※Odian Fig 3 -18 Entropy changes for all polymers are not so different. Sp= Sp- Sm Hp= Hp- Hm (–) value of Sp is higher if (–) , exothermic. Hanyang Univ.

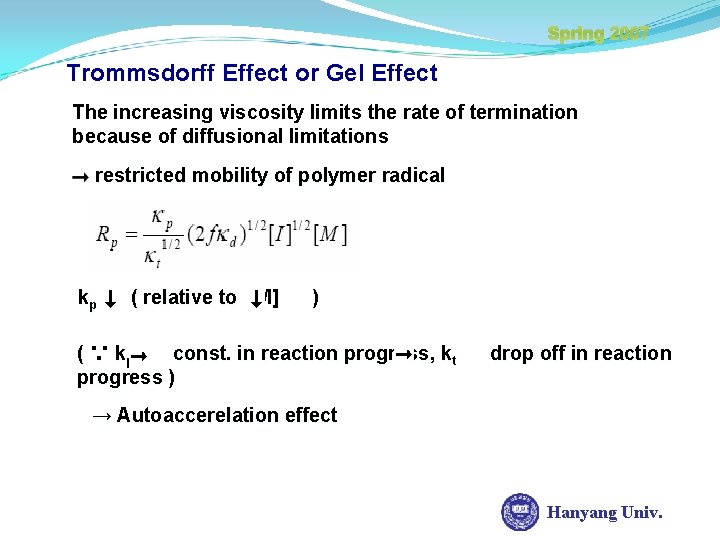
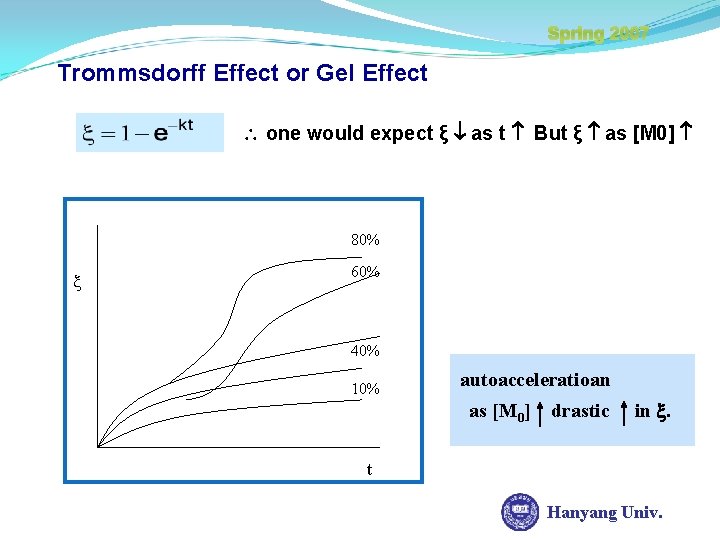
Spring 2007 Trommsdorff Effect or Gel Effect The increasing viscosity limits the rate of termination because of diffusional limitations restricted mobility of polymer radical kp ( relative to [M] ) ( ∵ kp const. in reaction progress, kt progress ) drop off in reaction → Autoaccerelation effect Hanyang Univ.

Spring 2007 Trommsdorff Effect or Gel Effect one would expect ξ as t But ξ as [M 0] 80% 60% 40% 10% autoacceleratioan as [M 0] drastic in . t Hanyang Univ.