Polymerization kinetics Stepwise polymerization any two monomers present

![• Michaelis-Menten equation can be obtained by plug the value of [ES] into • Michaelis-Menten equation can be obtained by plug the value of [ES] into](https://slidetodoc.com/presentation_image_h/d80a03252d1a37eec1064265d1e8494e/image-14.jpg)
- Slides: 17

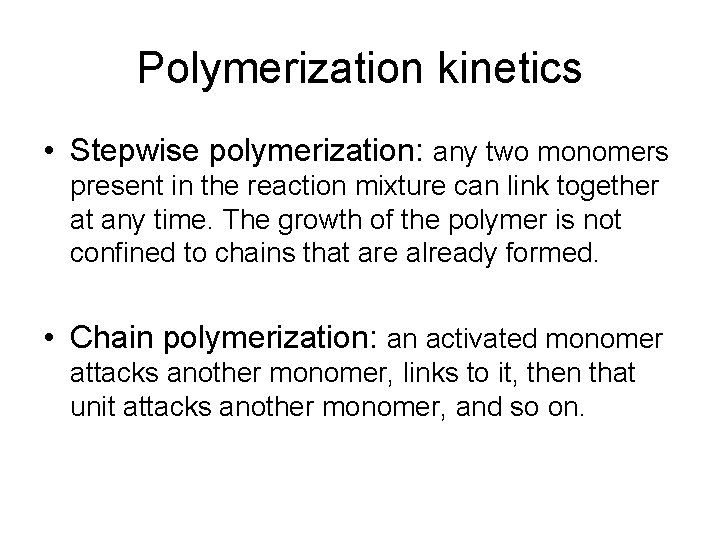
Polymerization kinetics • Stepwise polymerization: any two monomers present in the reaction mixture can link together at any time. The growth of the polymer is not confined to chains that are already formed. • Chain polymerization: an activated monomer attacks another monomer, links to it, then that unit attacks another monomer, and so on.

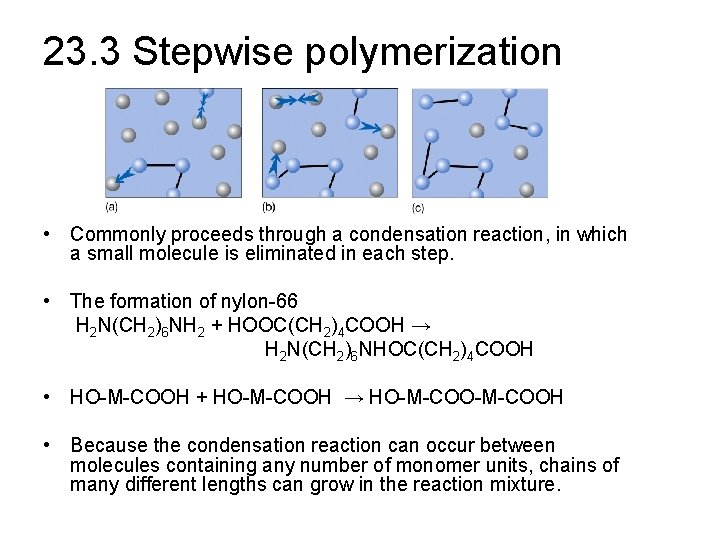
23. 3 Stepwise polymerization • Commonly proceeds through a condensation reaction, in which a small molecule is eliminated in each step. • The formation of nylon-66 H 2 N(CH 2)6 NH 2 + HOOC(CH 2)4 COOH → H 2 N(CH 2)6 NHOC(CH 2)4 COOH • HO-M-COOH + HO-M-COOH → HO-M-COOH • Because the condensation reaction can occur between molecules containing any number of monomer units, chains of many different lengths can grow in the reaction mixture.

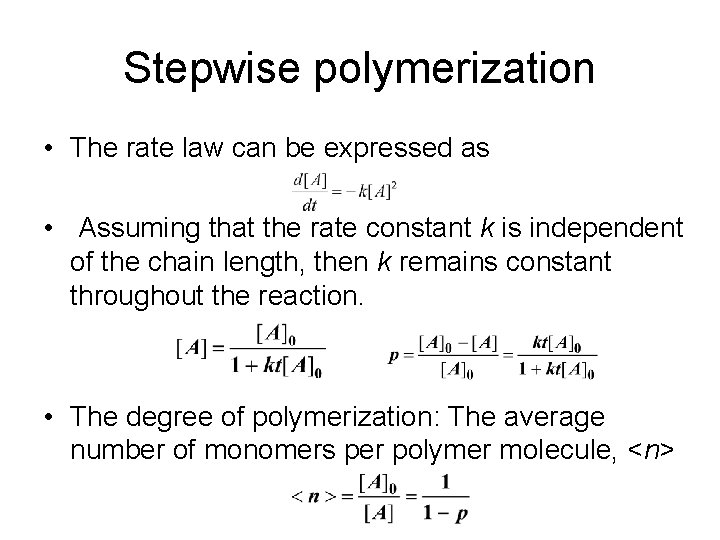
Stepwise polymerization • The rate law can be expressed as • Assuming that the rate constant k is independent of the chain length, then k remains constant throughout the reaction. • The degree of polymerization: The average number of monomers per polymer molecule, <n>

23. 4 Chain polymerization • Occurs by addition of monomers to a growing polymer, often by a radical chain process. • Rapid growth of an individual polymer chain for each activated monomer. • The addition polymerizations of ethene, methyl methacrylate, and styrene. • The rate of polymerization is proportional to the square root of the initiator concentration.

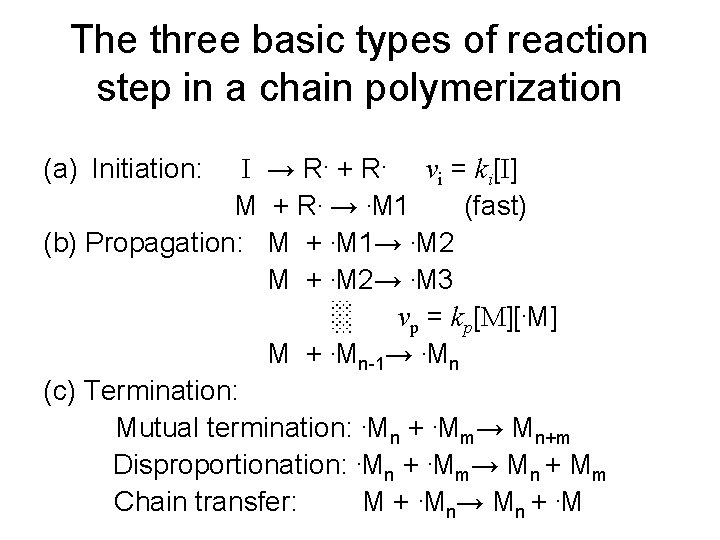
The three basic types of reaction step in a chain polymerization I → R. + R. vi = ki[I] M + R. →. M 1 (fast) (b) Propagation: M +. M 1→. M 2 M +. M 2→. M 3 ░ vp = kp[M][. M] M +. Mn-1→. Mn (c) Termination: Mutual termination: . Mn +. Mm→ Mn+m Disproportionation: . Mn +. Mm→ Mn + Mm Chain transfer: M +. Mn → M n +. M (a) Initiation:

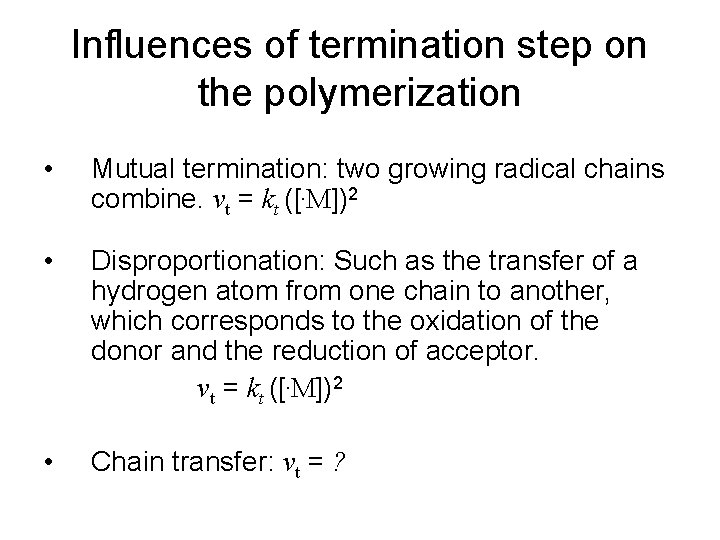
Influences of termination step on the polymerization • Mutual termination: two growing radical chains combine. vt = kt ([. M])2 • Disproportionation: Such as the transfer of a hydrogen atom from one chain to another, which corresponds to the oxidation of the donor and the reduction of acceptor. vt = kt ([. M])2 • Chain transfer: vt = ?

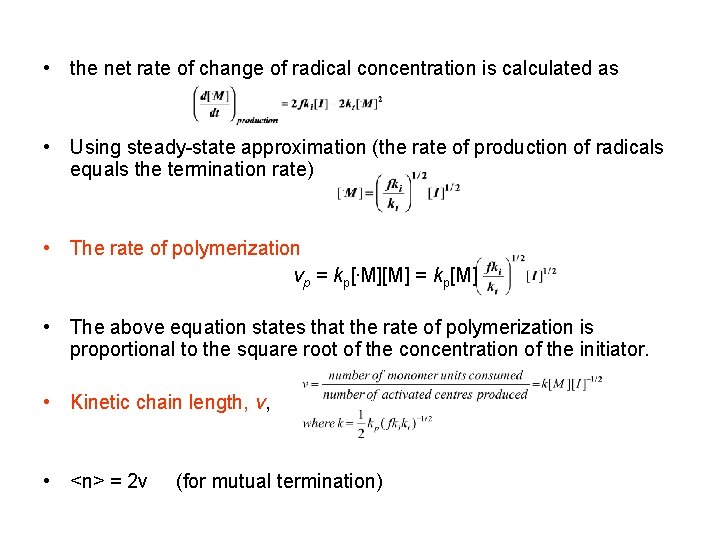
• the net rate of change of radical concentration is calculated as • Using steady-state approximation (the rate of production of radicals equals the termination rate) • The rate of polymerization vp = kp[. M][M] = kp[M] • The above equation states that the rate of polymerization is proportional to the square root of the concentration of the initiator. • Kinetic chain length, v, • <n> = 2 v (for mutual termination)

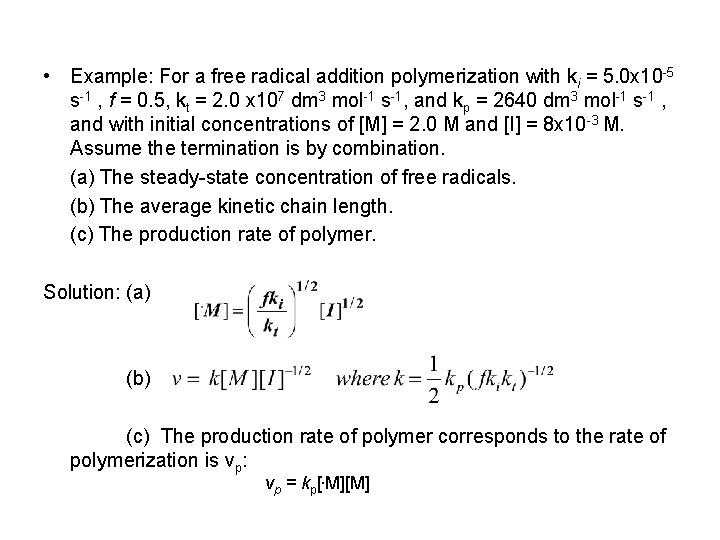
• Example: For a free radical addition polymerization with ki = 5. 0 x 10 -5 s-1 , f = 0. 5, kt = 2. 0 x 107 dm 3 mol-1 s-1, and kp = 2640 dm 3 mol-1 s-1 , and with initial concentrations of [M] = 2. 0 M and [I] = 8 x 10 -3 M. Assume the termination is by combination. (a) The steady-state concentration of free radicals. (b) The average kinetic chain length. (c) The production rate of polymer. Solution: (a) (b) (c) The production rate of polymer corresponds to the rate of polymerization is vp: vp = kp[. M][M]

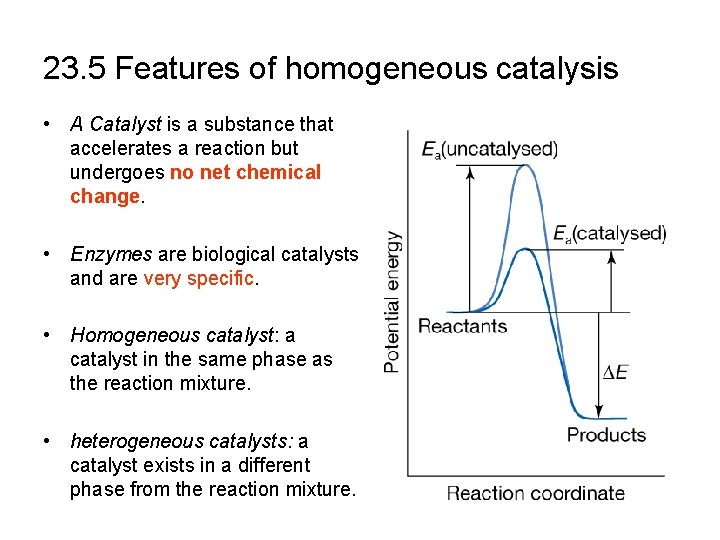
23. 5 Features of homogeneous catalysis • A Catalyst is a substance that accelerates a reaction but undergoes no net chemical change. • Enzymes are biological catalysts and are very specific. • Homogeneous catalyst: a catalyst in the same phase as the reaction mixture. • heterogeneous catalysts: a catalyst exists in a different phase from the reaction mixture.

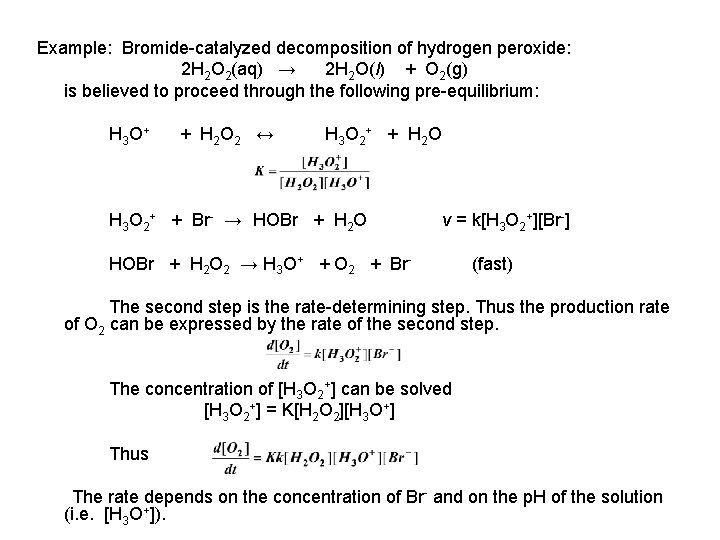
Example: Bromide-catalyzed decomposition of hydrogen peroxide: 2 H 2 O 2(aq) → 2 H 2 O(l) + O 2(g) is believed to proceed through the following pre-equilibrium: H 3 O + + H 2 O 2 ↔ H 3 O 2 + + H 2 O H 3 O 2+ + Br- → HOBr + H 2 O v = k[H 3 O 2+][Br-] HOBr + H 2 O 2 → H 3 O+ + O 2 + Br- (fast) The second step is the rate-determining step. Thus the production rate of O 2 can be expressed by the rate of the second step. The concentration of [H 3 O 2+] can be solved [H 3 O 2+] = K[H 2 O 2][H 3 O+] Thus The rate depends on the concentration of Br- and on the p. H of the solution (i. e. [H 3 O+]).

• Exercise 23. 4 b: Consider the acid-catalysed reaction (1) HA + H+ ↔ HAH+ k 1, k 1’ , both fast (2) HAH+ + B → BH+ + AH k 2, slow Deduce the rate law and show that it can be made independent of the specific term [H+] Solution:

23. 6 Enzymes Three principal features of enzyme-catalyzed reactions: 1. For a given initial concentration of substrate, [S]0, the initial rate of product formation is proportional to the total concentration of enzyme, [E]0. 2. For a given [E]0 and low values of [S]0, the rate of product formation is proportional to [S]0. 3. For a given [E]0 and high values of [S]0, the rate of product formation becomes independent of [S]0, reaching a maximum value known as the maximum velocity, vmax.

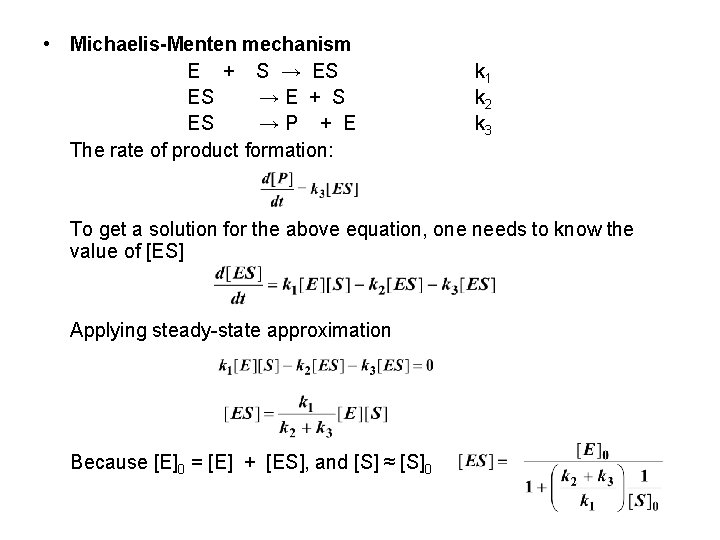
• Michaelis-Menten mechanism E + S → ES ES →E + S ES →P + E The rate of product formation: k 1 k 2 k 3 To get a solution for the above equation, one needs to know the value of [ES] Applying steady-state approximation Because [E]0 = [E] + [ES], and [S] ≈ [S]0
![MichaelisMenten equation can be obtained by plug the value of ES into • Michaelis-Menten equation can be obtained by plug the value of [ES] into](https://slidetodoc.com/presentation_image_h/d80a03252d1a37eec1064265d1e8494e/image-14.jpg)
• Michaelis-Menten equation can be obtained by plug the value of [ES] into the rate law of P: • Michaelis-Menten constant: KM can also be expressed as [E][S]/[ES]. • Analysis: 1. When [S]0 << KM, the rate of product formation is proportional to [S]0: 2. When [S]0 >> KM, the rate of product formation reaches its maximum value, which is independent of [S]0: v = vmax = k 3[E]0

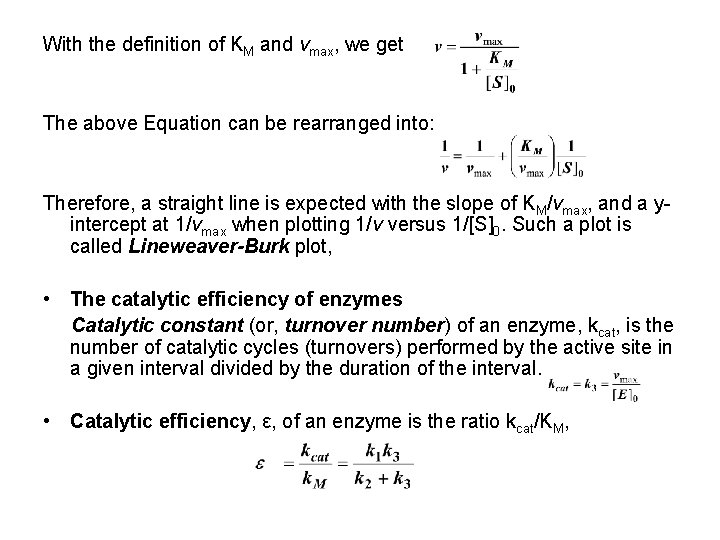
With the definition of KM and vmax, we get The above Equation can be rearranged into: Therefore, a straight line is expected with the slope of KM/vmax, and a yintercept at 1/vmax when plotting 1/v versus 1/[S]0. Such a plot is called Lineweaver-Burk plot, • The catalytic efficiency of enzymes Catalytic constant (or, turnover number) of an enzyme, kcat, is the number of catalytic cycles (turnovers) performed by the active site in a given interval divided by the duration of the interval. • Catalytic efficiency, ε, of an enzyme is the ratio kcat/KM,

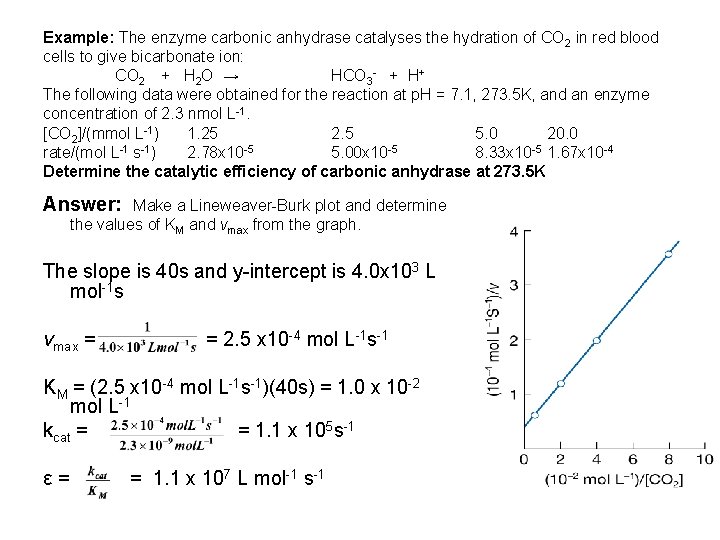
Example: The enzyme carbonic anhydrase catalyses the hydration of CO 2 in red blood cells to give bicarbonate ion: CO 2 + H 2 O → HCO 3 - + H+ The following data were obtained for the reaction at p. H = 7. 1, 273. 5 K, and an enzyme concentration of 2. 3 nmol L-1. [CO 2]/(mmol L-1) 1. 25 2. 5 5. 0 20. 0 rate/(mol L-1 s-1) 2. 78 x 10 -5 5. 00 x 10 -5 8. 33 x 10 -5 1. 67 x 10 -4 Determine the catalytic efficiency of carbonic anhydrase at 273. 5 K Answer: Make a Lineweaver-Burk plot and determine the values of KM and vmax from the graph. The slope is 40 s and y-intercept is 4. 0 x 103 L mol-1 s vmax = = 2. 5 x 10 -4 mol L-1 s-1 KM = (2. 5 x 10 -4 mol L-1 s-1)(40 s) = 1. 0 x 10 -2 mol L-1 kcat = = 1. 1 x 105 s-1 ε= = 1. 1 x 107 L mol-1 s-1

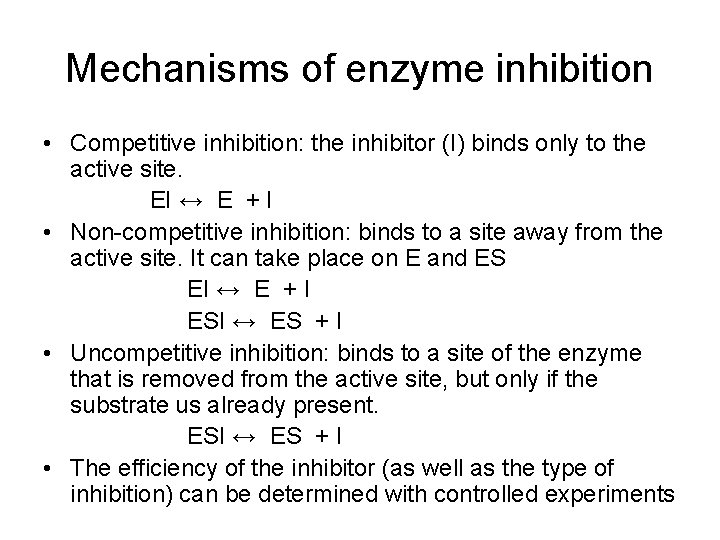
Mechanisms of enzyme inhibition • Competitive inhibition: the inhibitor (I) binds only to the active site. EI ↔ E + I • Non-competitive inhibition: binds to a site away from the active site. It can take place on E and ES EI ↔ E + I ESI ↔ ES + I • Uncompetitive inhibition: binds to a site of the enzyme that is removed from the active site, but only if the substrate us already present. ESI ↔ ES + I • The efficiency of the inhibitor (as well as the type of inhibition) can be determined with controlled experiments