Qualitative description of emulsion polymerization kinetics Suspension Polymerization


![Glasovergangstemperaturen Tg [°C] Polyvinylacetaat + 29 Polyvinylpropionaat +7 Poly(Veo. Va 10) -2 Polyvinylidenchloride + Glasovergangstemperaturen Tg [°C] Polyvinylacetaat + 29 Polyvinylpropionaat +7 Poly(Veo. Va 10) -2 Polyvinylidenchloride +](https://slidetodoc.com/presentation_image_h2/995e59c128b22138d1782f2f0f3dcf7d/image-7.jpg)
![Glasovergangstemperaturen Monomer: Tg [°C] Methyl. Ethyl n-Butyl 2 -Ethylhexyl - Acrylaat +8 - 22 Glasovergangstemperaturen Monomer: Tg [°C] Methyl. Ethyl n-Butyl 2 -Ethylhexyl - Acrylaat +8 - 22](https://slidetodoc.com/presentation_image_h2/995e59c128b22138d1782f2f0f3dcf7d/image-8.jpg)


- Slides: 11

Qualitative description of emulsion polymerization kinetics Suspension Polymerization Advantages Disadvantages * Heat control simple * Contamination with * Product directly stabilizing agent usable * Coagulation possible * Easy handling Applications Ion-exchange resins, polystyrene foam, PVC 1

Qualitative description of emulsion polymerization kinetics Antonio de Herrera Tordesillas described an application of natural latex in 1601. A ball game as part of a religious rite. 2

Emulsion Polymerization Advantages Disadvantages * Low viscosity even * Contamination of at high solid contents products with additives * Independent control * More complicated of rate and in case of water molecular-weight soluble monomers * Direct application of complete reactor contents 3

Applications of latices Paints-Construction * Paints / rough casting / heat insulation * Elastomeric coatings / primers * Additives for cement and concrete * Anti corrosives / wood coatings * Industrial coatings * Rheology modifiers * Roof coatings * Fillers and levelling powders * Varnishes Paper * Binders for rubbed paper * Boxes and wallpaper Other * Teflon * Elastomers Glues-Adhesives * Wood glues / adhesives for furniture laminates * Adhesives for floor-, wall- and ceiling materials * Packaging- and lamination glues * Adhesion- and contact glues * Leather fibres * Glues in powder form * Structural Adhesives * Contact Adhesives Textiles * Carpet backside coatings * Fleece binder * Spunbond / textile coating * Equipment of technical textiles * Pressure binder /flocculating glue Source: Clariant 4

Objective Ho w to in flu en ce ? Process Molecular Microstructure & Morphology What is the relation ? Properties 5

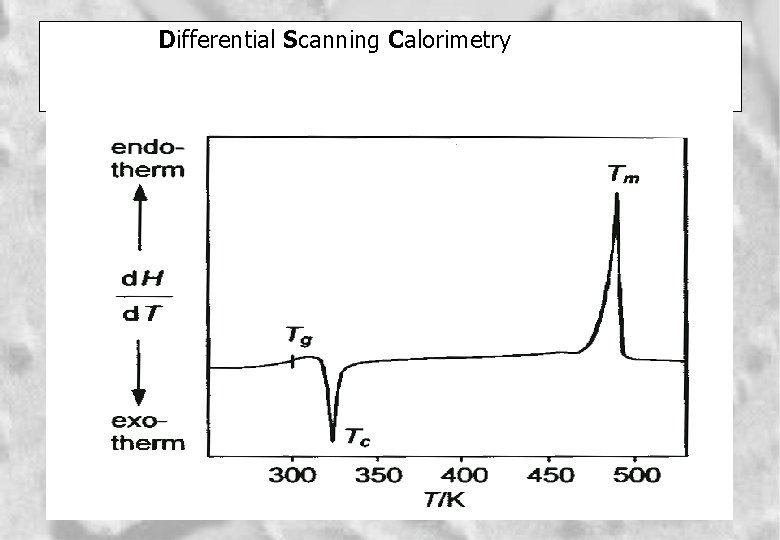
Differential Scanning Calorimetry 6
![Glasovergangstemperaturen Tg C Polyvinylacetaat 29 Polyvinylpropionaat 7 PolyVeo Va 10 2 Polyvinylidenchloride Glasovergangstemperaturen Tg [°C] Polyvinylacetaat + 29 Polyvinylpropionaat +7 Poly(Veo. Va 10) -2 Polyvinylidenchloride +](https://slidetodoc.com/presentation_image_h2/995e59c128b22138d1782f2f0f3dcf7d/image-7.jpg)
Glasovergangstemperaturen Tg [°C] Polyvinylacetaat + 29 Polyvinylpropionaat +7 Poly(Veo. Va 10) -2 Polyvinylidenchloride + 80 Polyacrylonitril + 100 Polyethyleen (-125) Polystyreen + 100 Polymethylmethacrylaat +105 Poly 2 -ethyl-hexylacrylaat -85 Poly n-butylacrylaat -54 Polyacrylzuur (kristallijn) + 166 Poly methacrylzuur Poly acrylamide (kristallijn) + 185 + 153 Polyhydroxy-ethyl-methacrylaat + 55 7
![Glasovergangstemperaturen Monomer Tg C Methyl Ethyl nButyl 2 Ethylhexyl Acrylaat 8 22 Glasovergangstemperaturen Monomer: Tg [°C] Methyl. Ethyl n-Butyl 2 -Ethylhexyl - Acrylaat +8 - 22](https://slidetodoc.com/presentation_image_h2/995e59c128b22138d1782f2f0f3dcf7d/image-8.jpg)
Glasovergangstemperaturen Monomer: Tg [°C] Methyl. Ethyl n-Butyl 2 -Ethylhexyl - Acrylaat +8 - 22 - 54 - 85 Methyl Ethyl n-Butyl 2 -Ethylhexyl Decyl n-Butyl iso-Butyl sek. -Butyl tert. -Butyl - Methacrylaat - Methacrylaat - Acrylaat + 105 + 65 + 20 - 10 - 70 - 54 - 43 - 20 + 41 n-Butyl iso-Butyl sek. -Butyl tert. -Butyl - Methacrylaat + 20 + 48 + 60 + 107 8

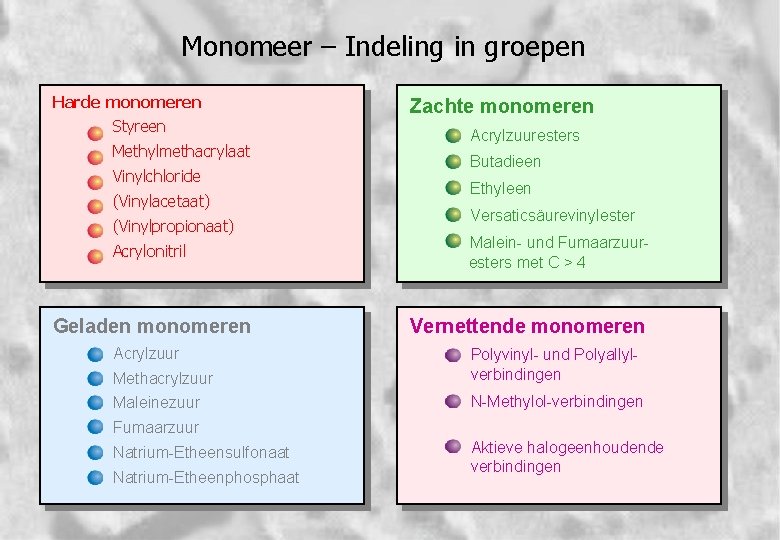
Monomeer – Indeling in groepen Harde monomeren Styreen Methylmethacrylaat Vinylchloride (Vinylacetaat) (Vinylpropionaat) Acrylonitril Geladen monomeren Acrylzuur Zachte monomeren Acrylzuuresters Butadieen Ethyleen Versaticsäurevinylester Malein- und Fumaarzuuresters met C > 4 Vernettende monomeren Methacrylzuur Polyvinyl- und Polyallylverbindingen Maleinezuur N-Methylol-verbindingen Fumaarzuur Natrium-Etheensulfonaat Natrium-Etheenphosphaat Aktieve halogeenhoudende verbindingen 9

Spherulieten in de microstructuur van een polymeer. 10

De 3 M aspecten van de PE proef. Links de toepassing, een beker (macro), met in het midden de morfologie van dit polymeer (meso). Rechts de atomaire ketens zoals die in het polymeer voorkomen (micro). 11