Polymerization Reactions What is Polymerization t Polymerization is


- Slides: 15

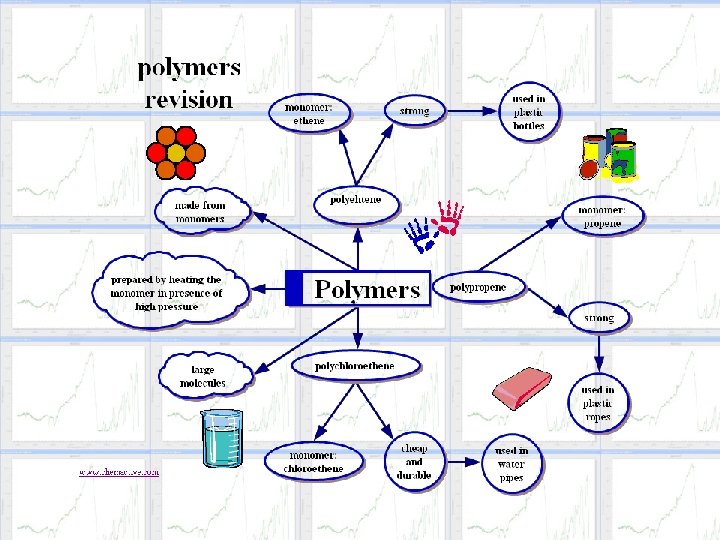
Polymerization Reactions


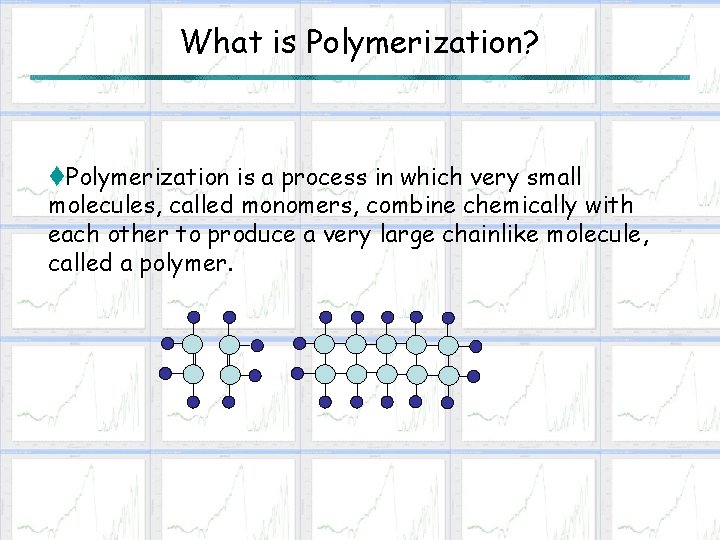
What is Polymerization? t. Polymerization is a process in which very small molecules, called monomers, combine chemically with each other to produce a very large chainlike molecule, called a polymer.



The First Polymerization t. The first synthetic polymer was discovered in 1909 by Leo Backeland, a Belgian-born US Chemist. t. It was marketed under the trademark Bakelite, which was made from phenol and formaldehyde. t. It became an important plastic and resin for adhesives, and paints.

Polymerization Types t. There are two main types of Polymerization reactions: t. Addition 2+2 t. Condensation Think water, although that is not always the case

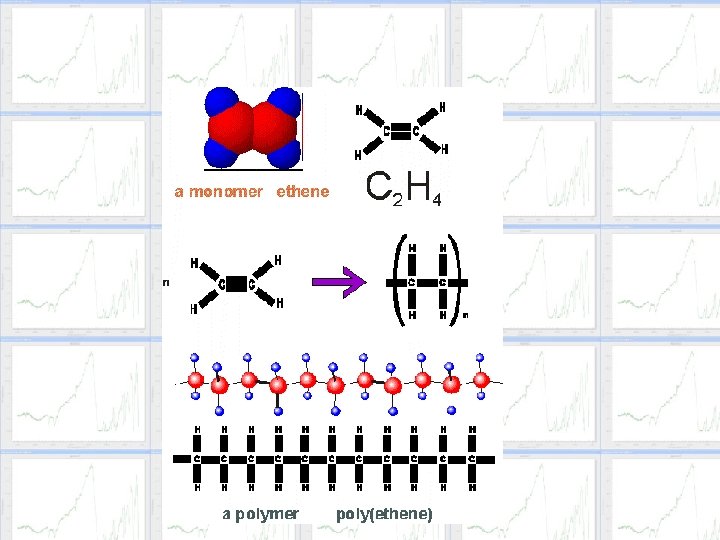
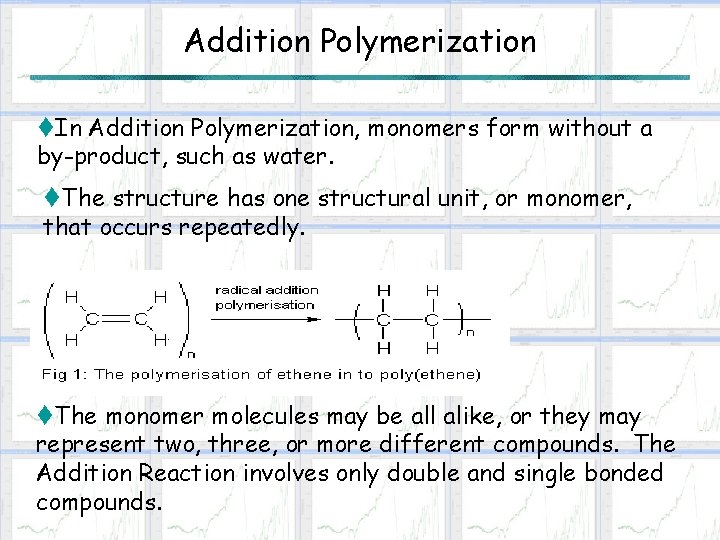
Addition Polymerization t. In Addition Polymerization, monomers form without a by-product, such as water. t. The structure has one structural unit, or monomer, that occurs repeatedly. t. The monomer molecules may be all alike, or they may represent two, three, or more different compounds. The Addition Reaction involves only double and single bonded compounds.

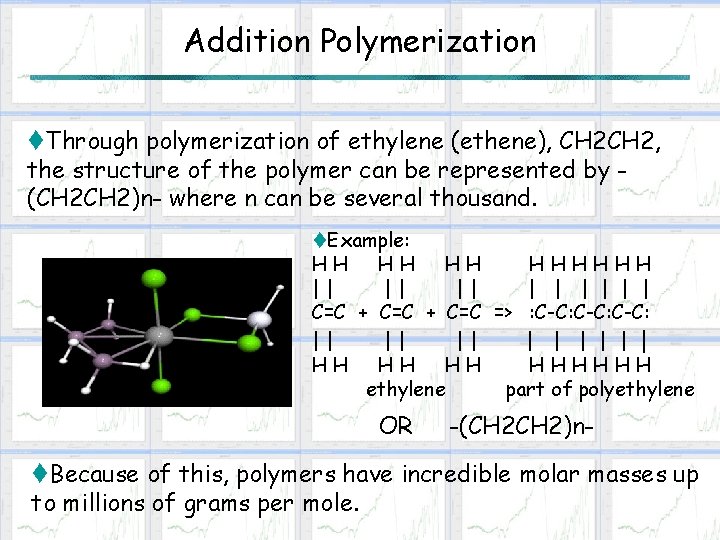
Addition Polymerization t. Through polymerization of ethylene (ethene), CH 2, the structure of the polymer can be represented by (CH 2)n- where n can be several thousand. t. Example: HH HHHHHH || || || | | | C=C + C=C => : C-C: || || || | | | HH HHHHHH ethylene part of polyethylene OR -(CH 2)n- t. Because of this, polymers have incredible molar masses up to millions of grams per mole.

Addition Polymerization t. The elastic property of rubber is due to the flexibility of the long chain molecules. t. In 1839, American chemist Charles Goodyear discovered that natural rubber could be cross-linked with sulfur. This would prevent the long chains from slipping. t. This process was known as vulcanization. t. It paved that way for many practical and commercial usage, including using vulcanized rubber for automobile tires.

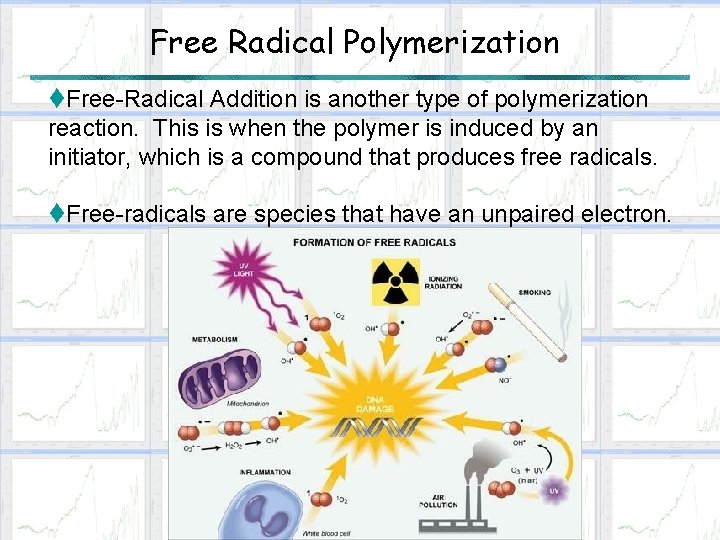
Free Radical Polymerization t. Free-Radical Addition is another type of polymerization reaction. This is when the polymer is induced by an initiator, which is a compound that produces free radicals. t. Free-radicals are species that have an unpaired electron.

Condensation Polymerization t. In condensation polymerization, two functional groups of two different monomer molecules are joined together which produces a small molecule such as water. Remember the water? t. The monomers bond at where the hydrogen atoms were taken out to produce water. In order to become a condensed polymer, the monomer molecules must have at least two functional groups.

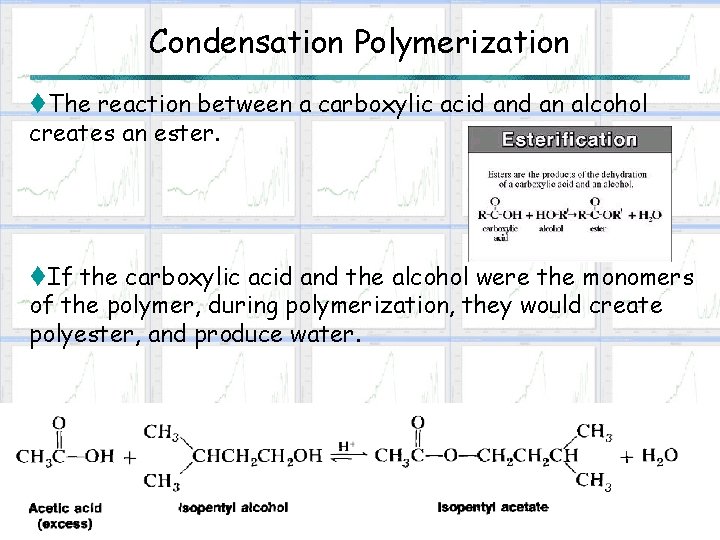
Condensation Polymerization t. The reaction between a carboxylic acid an alcohol creates an ester. t. If the carboxylic acid and the alcohol were the monomers of the polymer, during polymerization, they would create polyester, and produce water.

Condensation Polymerization t. Example of condensation polymerization: Curing of concrete t. There are several types of materials that form by Condensation Polymerization, including nylon, polyesters, rayon, and spandex.
