Sources of Radiation Natural Terrestrial Radiation IAEA Day












- Slides: 27

Sources of Radiation Natural Terrestrial Radiation IAEA Day 3 – Lecture 7 1

OBJECTIVE To discuss about the natural terrestrial radiation, radioactive decay chains, important radionuclides, radon and its significance, NORM, TENORM and NARM IAEA 2

Content Ø Terrestrial decay chains Ø Important radionuclides Ø Radon and its health significance Ø NORM Ø TENORM Ø NARM IAEA 3

Radioactivity in Nature Ø Primordial – existing since the creation of the Earth Ø Cosmogenic – formed as a result of cosmic ray interactions Ø Human produced – enhanced or formed due to human actions IAEA 4

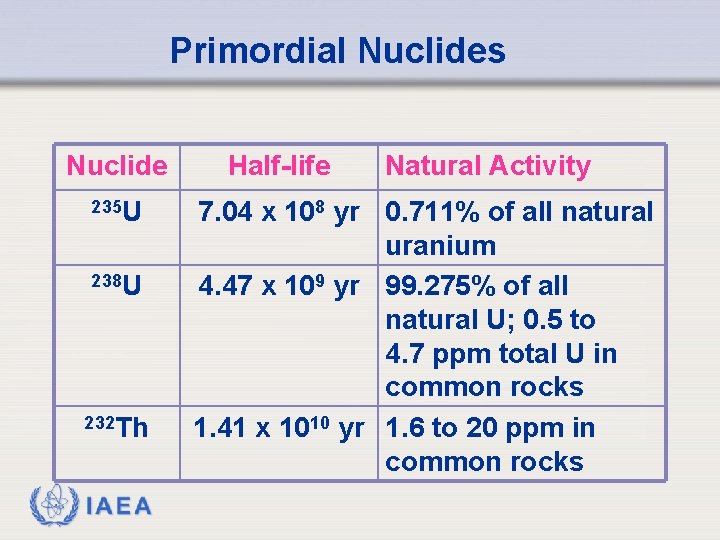
Primordial Nuclides Nuclide 235 U 238 U 232 Th IAEA Half-life Natural Activity 7. 04 x 108 yr 0. 711% of all natural uranium 4. 47 x 109 yr 99. 275% of all natural U; 0. 5 to 4. 7 ppm total U in common rocks 1. 41 x 1010 yr 1. 6 to 20 ppm in common rocks

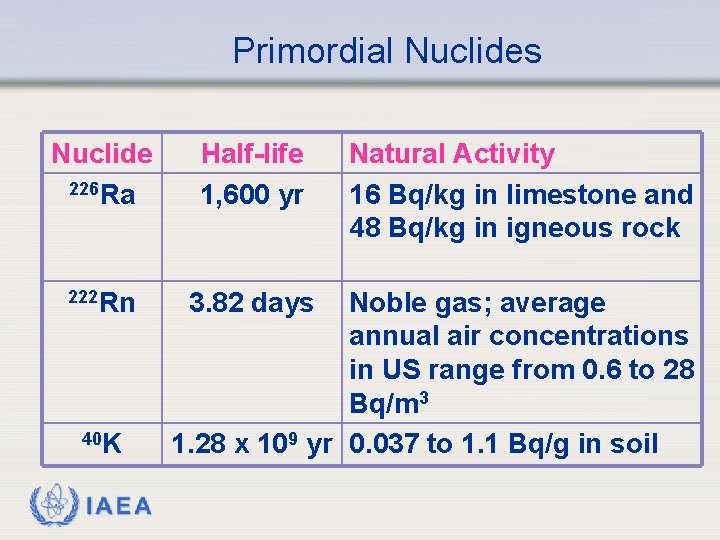
Primordial Nuclides Nuclide 226 Ra Half-life 1, 600 yr 222 Rn 3. 82 days 40 K IAEA Natural Activity 16 Bq/kg in limestone and 48 Bq/kg in igneous rock Noble gas; average annual air concentrations in US range from 0. 6 to 28 Bq/m 3 1. 28 x 109 yr 0. 037 to 1. 1 Bq/g in soil

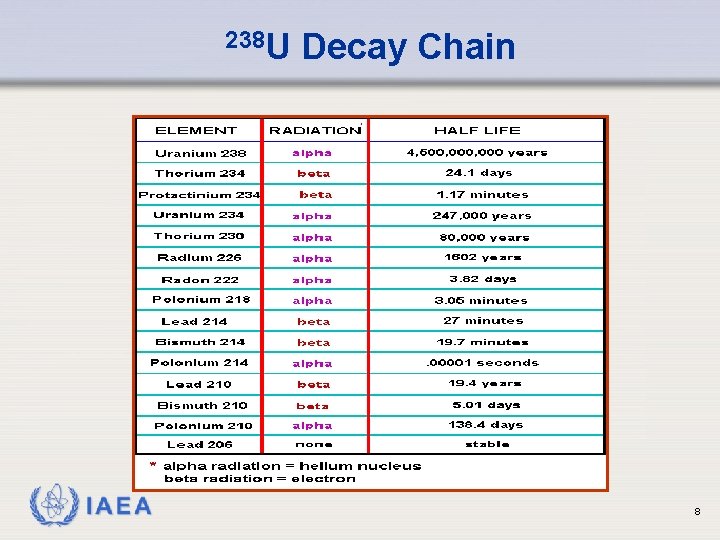
Background Radiation Ø There are three decay “chains” that occur in nature: Ø the uranium series, beginning with 238 U Ø the thorium series, which originates with 232 Th Ø the actinium series, which originates with 235 U Ø Once upon a time there was also a neptunium series, which originated with 241 Pu, that has a half-life of only 14 years. The only remaining member of this series is 209 Bi with a half-life of 2 E 18 years. IAEA 7

238 U IAEA Decay Chain 8

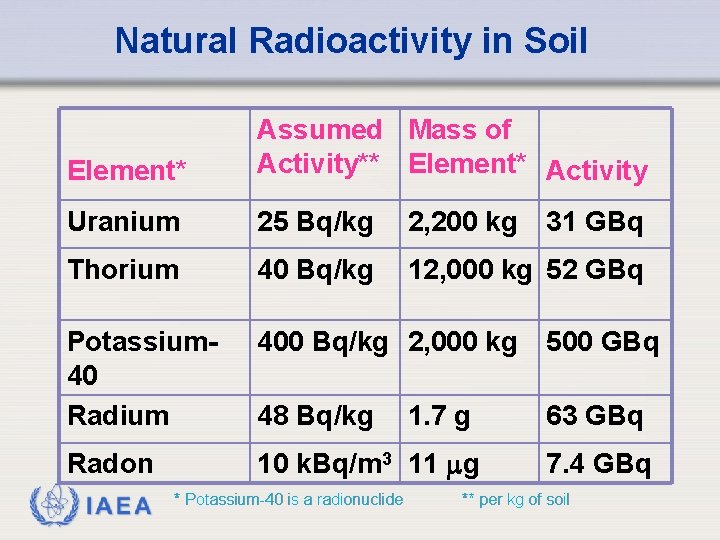
Natural Radioactivity in Soil Element* Assumed Mass of Activity** Element* Activity Uranium 25 Bq/kg 2, 200 kg Thorium 40 Bq/kg 12, 000 kg 52 GBq Potassium 40 Radium 400 Bq/kg 2, 000 kg 500 GBq 48 Bq/kg 63 GBq Radon 10 k. Bq/m 3 11 g IAEA * Potassium-40 is a radionuclide 1. 7 g 31 GBq 7. 4 GBq ** per kg of soil

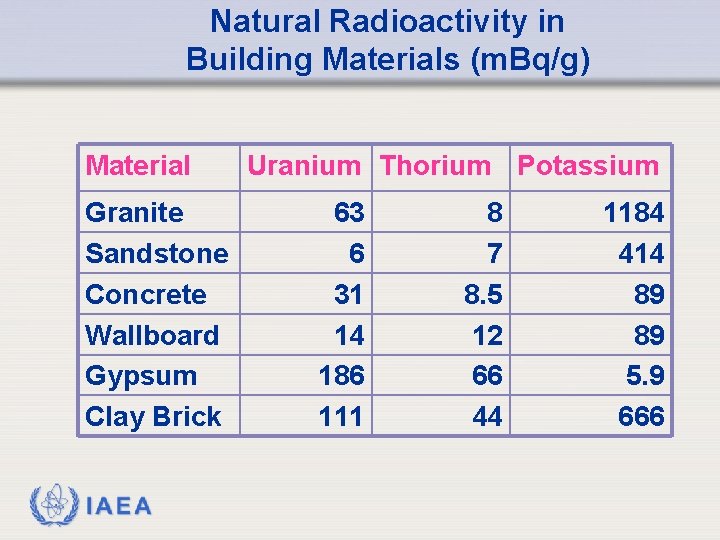
Natural Radioactivity in Building Materials (m. Bq/g) Material Granite Sandstone Concrete Wallboard Gypsum Clay Brick IAEA Uranium Thorium Potassium 63 6 31 14 186 111 8 7 8. 5 12 66 44 1184 414 89 89 5. 9 666

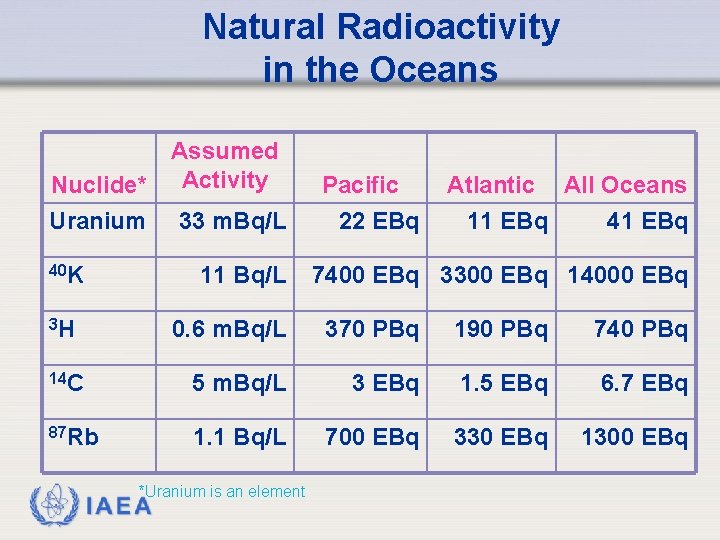
Natural Radioactivity in the Oceans Nuclide* Uranium 40 K Assumed Activity 33 m. Bq/L 11 Bq/L 3 H Pacific 22 EBq Atlantic All Oceans 11 EBq 41 EBq 7400 EBq 3300 EBq 14000 EBq 0. 6 m. Bq/L 370 PBq 190 PBq 740 PBq 14 C 5 m. Bq/L 3 EBq 1. 5 EBq 6. 7 EBq 87 Rb 1. 1 Bq/L 700 EBq 330 EBq 1300 EBq IAEA *Uranium is an element

Background Radiation - Radon Ø Radon is a noble gas (also called “inert”). Ø Radon is chemically like other members of this group of the periodic table, namely He, Ne, Ar, Kr, and Xe, gas. The noble gases do not readily form compounds due to their stable electron shell configuration. With the exception of helium, they all have 8 electrons in their outer shell (ns 2 np 6 for you chemists in the audience). IAEA 12

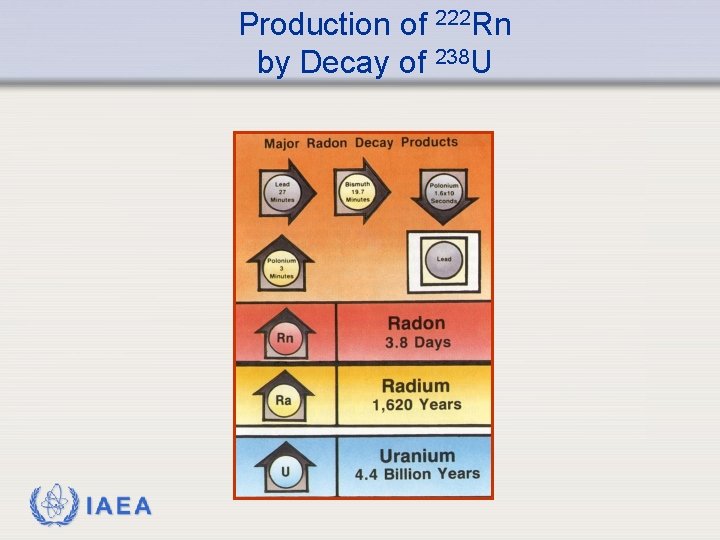
Background Radiation - Radon Ø The average dose from radon-222 (222 Rn) is approximately 2000 Sv per year. Radon is an alpha emitter. Many of the radon decay products are also alpha emitters. Ø Radon is produced from the radioactive decay of 238 U an isotope of uranium which is naturally present in the environment. In fact, in some areas of the western US, the concentration of natural uranium is high enough that is it mined to provide a source of uranium for reactors. IAEA 13

Production of 222 Rn by Decay of 238 U IAEA

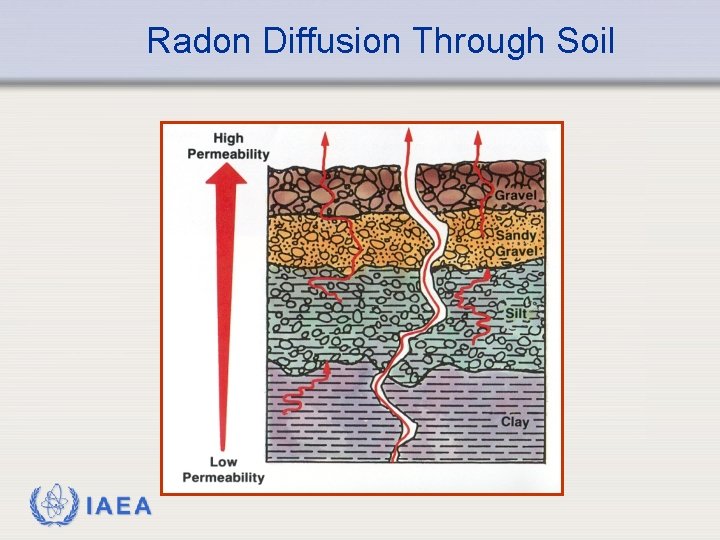
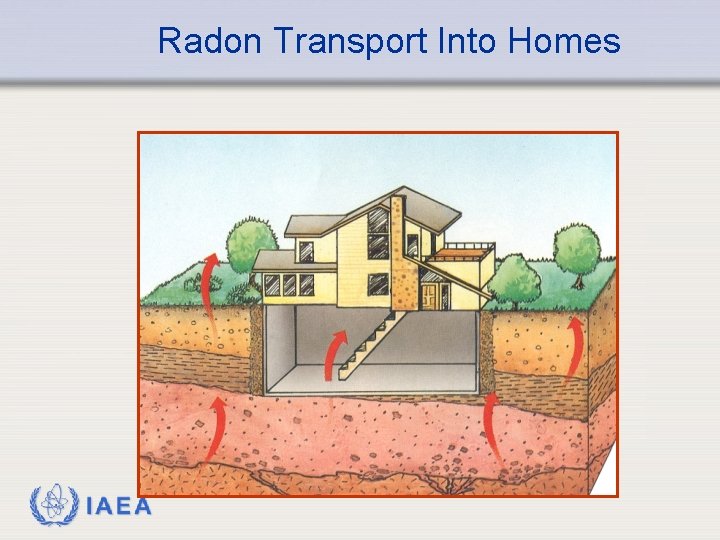
Background Radiation - Radon Ø Just as helium can diffuse through a balloon, radon can diffuse through the soil and foundations of homes. The diffusion is greater when the soil has low moisture content. Ø Radon is a radiological hazard because the decay products are alpha emitters. Since they are formed by emission of alpha particles, the resulting decay products have an electrostatic charge and are attracted to particulates in the air. These may become deposited in the lung. These particles then decay by alpha emission, which results in a dose to the lung. IAEA 15

Radon Diffusion Through Soil IAEA

Radon Transport Into Homes IAEA

Background Radiation - Radon Ø The concern is really not with the radioactive decay of radon, but with the radon progeny (also called “daughter products”), that are produced when radon decays. Since radon decays by alpha emission, the radon “daughters” have a ++ charge and so are electrostatically attached to particulates in the air. These decay products are also radioactive, and many decay by alpha particle emission. Ø The alpha particle energy delivers a dose to the lung where the particles are deposited. The dose to the lung is attributed to the development of lung cancer for uranium miners (hence the regulatory limits for radon and radon daughter concentrations). IAEA 18

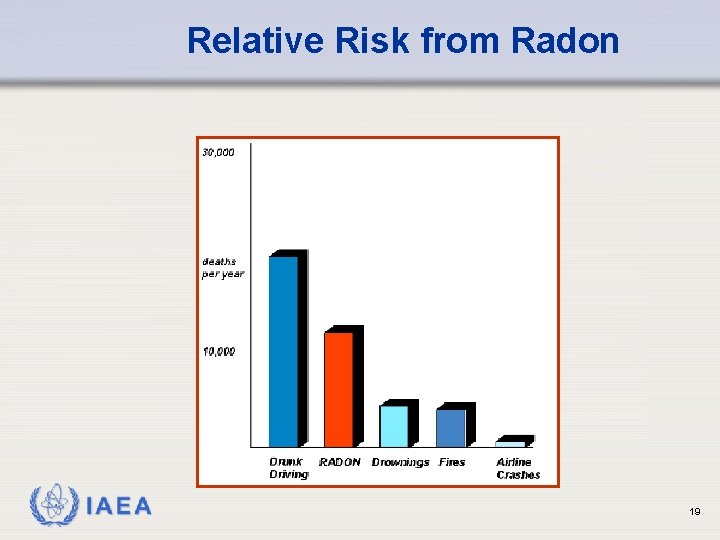
Relative Risk from Radon IAEA 19

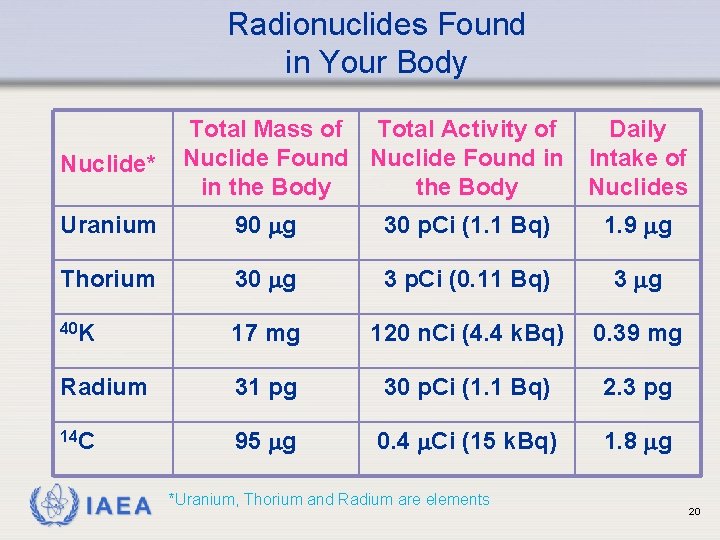
Radionuclides Found in Your Body Nuclide* Total Mass of Total Activity of Daily Nuclide Found in Intake of in the Body Nuclides Uranium 90 g 30 p. Ci (1. 1 Bq) 1. 9 g Thorium 30 g 3 p. Ci (0. 11 Bq) 3 g 40 K 17 mg 120 n. Ci (4. 4 k. Bq) 0. 39 mg Radium 31 pg 30 p. Ci (1. 1 Bq) 2. 3 pg 14 C 95 g 0. 4 Ci (15 k. Bq) 1. 8 g IAEA *Uranium, Thorium and Radium are elements 20

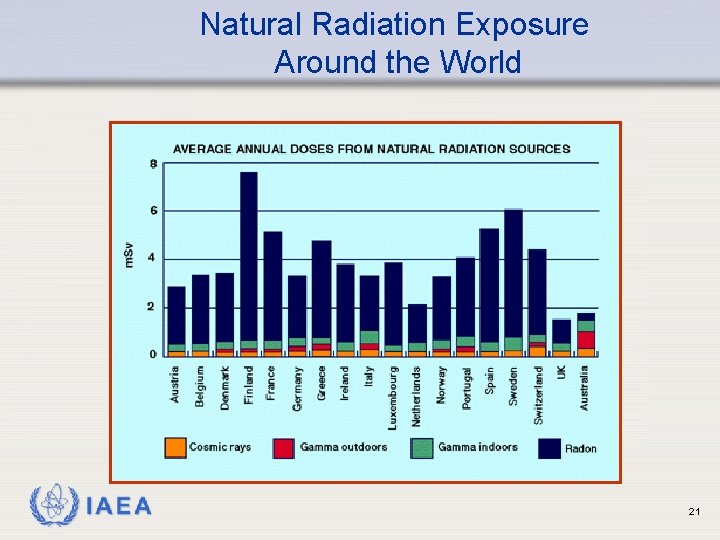
Natural Radiation Exposure Around the World IAEA 21

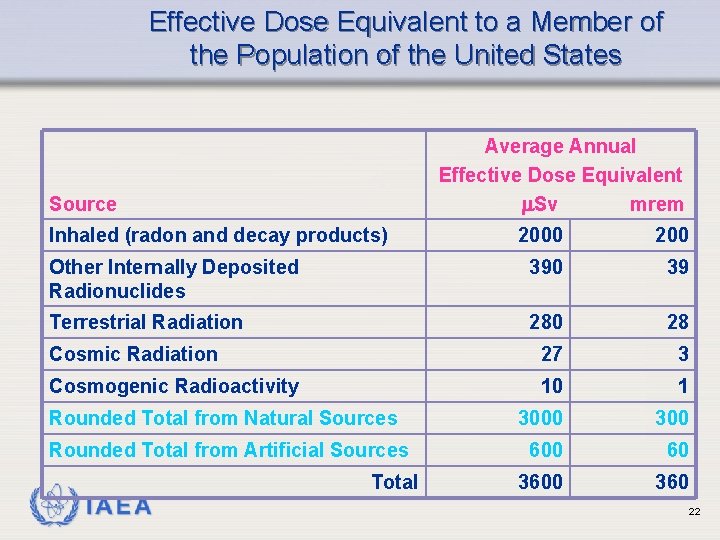
Effective Dose Equivalent to a Member of the Population of the United States Average Annual Effective Dose Equivalent Sv mrem Source Inhaled (radon and decay products) 2000 200 Other Internally Deposited Radionuclides 390 39 Terrestrial Radiation 280 28 Cosmic Radiation 27 3 Cosmogenic Radioactivity 10 1 Rounded Total from Natural Sources 3000 300 Rounded Total from Artificial Sources 600 60 360 IAEA Total 22

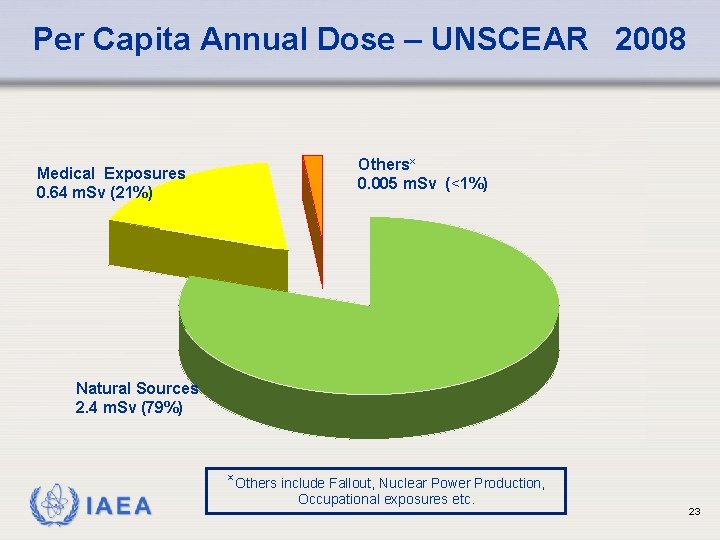
Per Capita Annual Dose – UNSCEAR 2008 Medical Exposures 0. 64 m. Sv (21%) Others× 0. 005 m. Sv (<1%) Natural Sources 2. 4 m. Sv (79%) ×Others include Fallout, Nuclear Power Production, IAEA Occupational exposures etc. 23

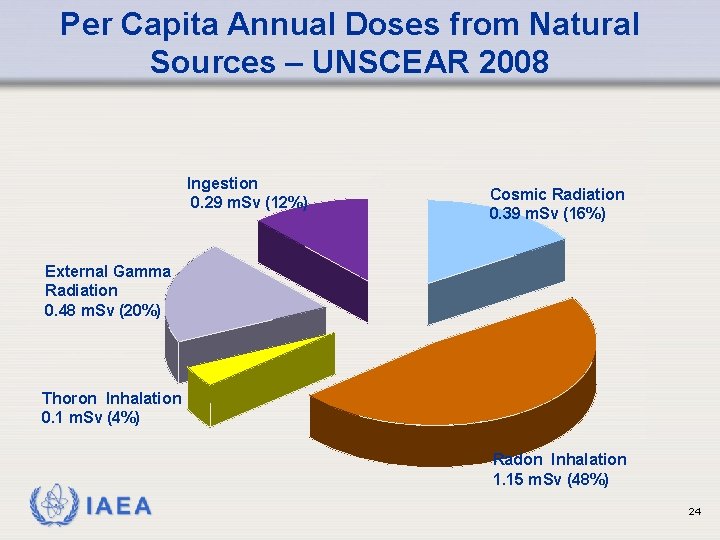
Per Capita Annual Doses from Natural Sources – UNSCEAR 2008 Ingestion 0. 29 m. Sv (12%) Cosmic Radiation 0. 39 m. Sv (16%) External Gamma Radiation 0. 48 m. Sv (20%) Thoron Inhalation 0. 1 m. Sv (4%) Radon Inhalation 1. 15 m. Sv (48%) IAEA 24

NORM and NARM Ø NORM Naturally Occurring Radioactive Material Ø TENORM Naturally Occurring Radioactive Material enhanced by processing is called TENORM Ø NARM Naturally Occurring and Accelerator-Produced Radioactive Material IAEA 25

Summary Ø Natural terrestrial radiation was discussed Ø Important radionuclides and their distribution was discussed Ø Radon in particular and its significance was discussed Ø NORM, TENORM and NARM were defined IAEA 26

Where to Get More Information Ø Cember, H. , Johnson, T. E, Introduction to Health Physics, 4 th Edition, Mc. Graw-Hill, New York (2009) Ø UNSCEAR, Sources and Effects of Ionizing Radiation, 2008 Report to the General Assembly with Scientific Annexes, United Nations, New York, 2008 Ø International Atomic Energy Agency, Postgraduate Educational Course in Radiation Protection and the Safety of Radiation Sources(PGEC), Training Course Series 18, IAEA, Vienna (2002) IAEA 27
 Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Nuclear energy facts
Nuclear energy facts Day 1 day 2 day 817
Day 1 day 2 day 817 Print sources and web sources
Print sources and web sources Water resources important
Water resources important Sújb
Sújb Ionizing radiation sources
Ionizing radiation sources Iaea ssr-5
Iaea ssr-5 Saris iaea
Saris iaea Iaea livechart
Iaea livechart Intouch plus iaea
Intouch plus iaea Part5ds
Part5ds Rtc protection film
Rtc protection film Iaea
Iaea Iaea
Iaea Stefano monti iaea
Stefano monti iaea Iaea
Iaea Iaea
Iaea Iaea
Iaea Iaea
Iaea Iaea
Iaea Gsr part 7
Gsr part 7 Iaea pcmf
Iaea pcmf Film badge dosimeter
Film badge dosimeter Iaea
Iaea Integrated management systems training north america
Integrated management systems training north america Natural source of heat
Natural source of heat Indeks fluorosis
Indeks fluorosis