SOURCES OF HEAT A NATURAL SOURCES 1 The


















- Slides: 44


SOURCES OF HEAT A. NATURAL SOURCES 1. The sun 2. The interior of the Earth B. ARTIFICIAL SOURCES 1. Chemical Action 2. Mechanical Energy 3. Electrical Energy 4. Nuclear Energy

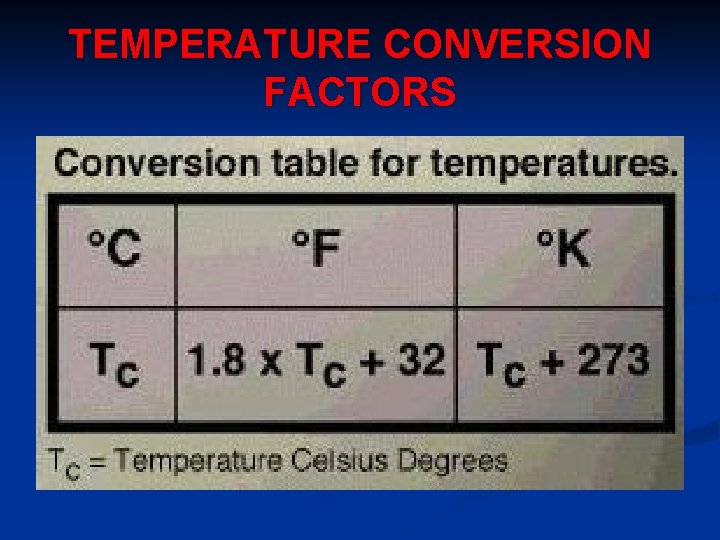
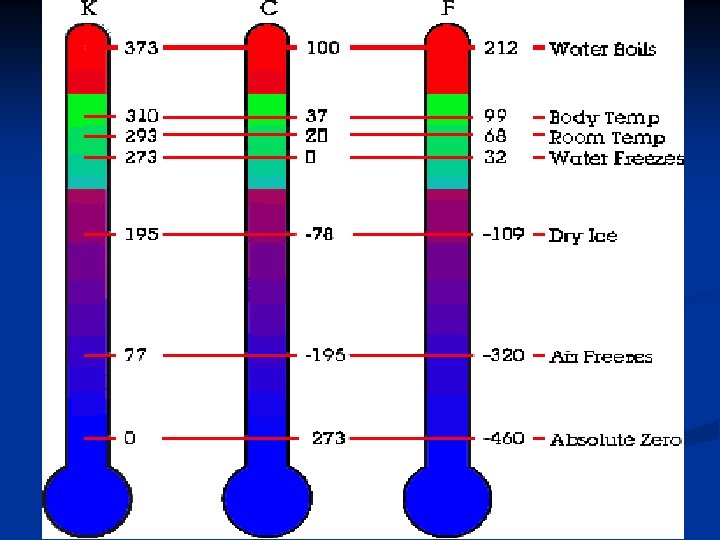
UNITS FOR TEMPERATURE 1. Fahrenheit Scale (o. F) – Fahrenheit temperature scale is a scale based on 32 for the freezing point of water and 212 for the boiling point of water, the interval between the two being divided into 180 parts. The 18 th-century German physicist Daniel Gabriel Fahrenheit originally took as the zero of his scale the temperature of an equal ice-salt mixture and selected the value of 98. 6 for normal body temperature.

2. Celsius Scale (o. C) - is based on 0 for the freezing point of water and 100 for the boiling point of water. Invented in 1742 by the Swedish astronomer Anders Celsius, it is sometimes called the centigrade scale because of the 100 -degree interval between the defined points.

3. Kelvin (K) - Kelvin temperature scale is the base unit of thermodynamic temperature measurement in the International System (SI) of measurement. It is defined as 1/ 273. 16 of the triple point (equilibrium among the solid, liquid, and gaseous phases) of pure water. Zero point absolute zero, theoretical temperature at which the molecules of a substance have the lowest energy. The kelvin has the same magnitude as the degree Celsius.

TEMPERATURE CONVERSION FACTORS


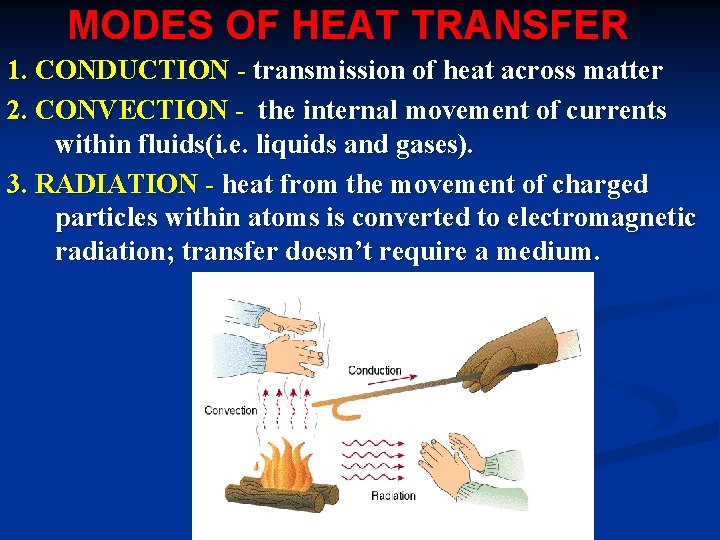
MODES OF HEAT TRANSFER 1. CONDUCTION - transmission of heat across matter 2. CONVECTION - the internal movement of currents within fluids(i. e. liquids and gases). 3. RADIATION - heat from the movement of charged particles within atoms is converted to electromagnetic radiation; transfer doesn’t require a medium.

Heat Transfer Heat always moves from a warmer place to a cooler place. Hot objects in a cooler room will cool to room temperature. Cold objects in a warmer room will heat up to room temperature.

Question If a cup of coffee and a red popsicle were left on the table in this room what would happen to them? Why? The cup of coffee will cool until it reaches room temperature. The popsicle will melt and then the liquid will warm to room temperature.

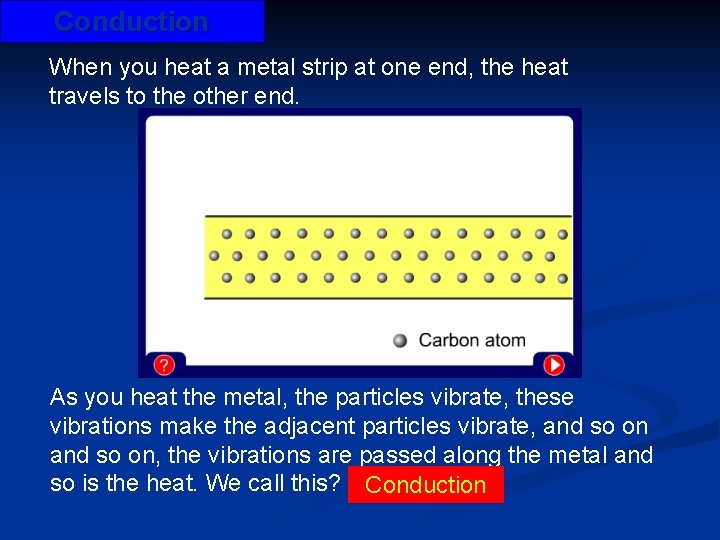
Conduction When you heat a metal strip at one end, the heat travels to the other end. As you heat the metal, the particles vibrate, these vibrations make the adjacent particles vibrate, and so on, the vibrations are passed along the metal and so is the heat. We call this? Conduction

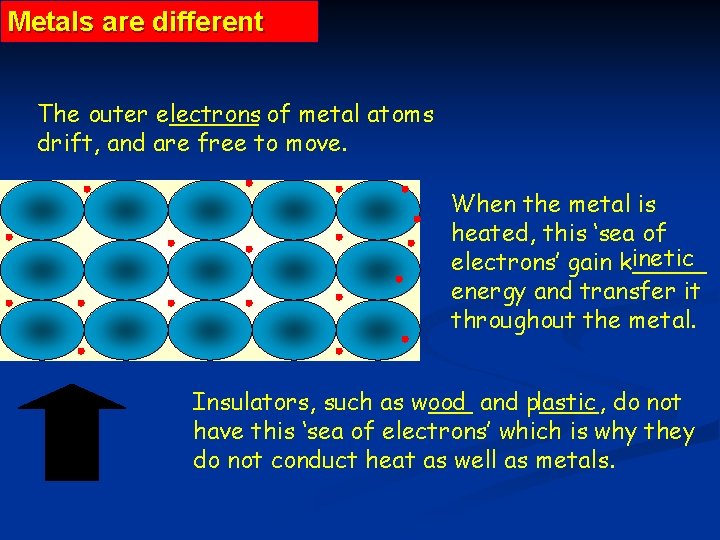
Metals are different The outer e______ lectrons of metal atoms drift, and are free to move. When the metal is heated, this ‘sea of inetic electrons’ gain k_____ energy and transfer it throughout the metal. Insulators, such as w___ ood and p____, lastic do not have this ‘sea of electrons’ which is why they do not conduct heat as well as metals.

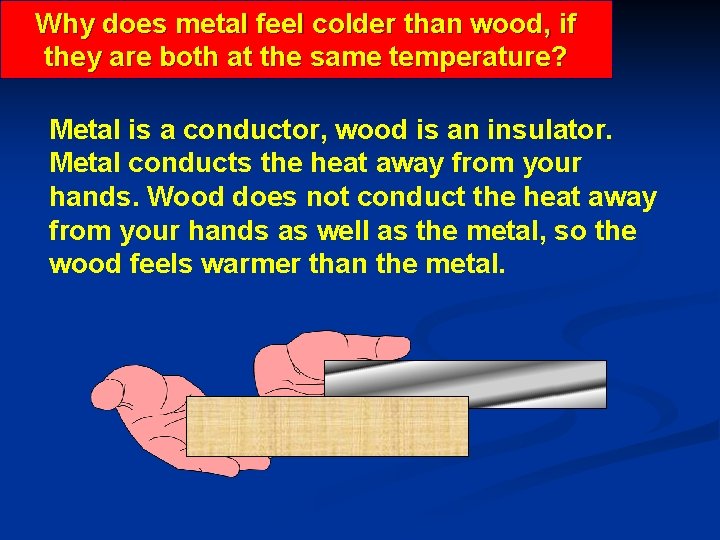
Why does metal feel colder than wood, if they are both at the same temperature? Metal is a conductor, wood is an insulator. Metal conducts the heat away from your hands. Wood does not conduct the heat away from your hands as well as the metal, so the wood feels warmer than the metal.

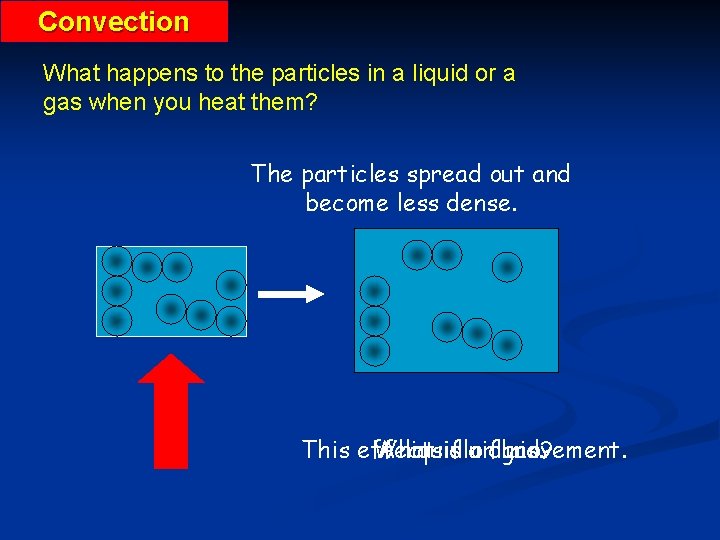
Convection What happens to the particles in a liquid or a gas when you heat them? The particles spread out and become less dense. This effects What A liquid isfluid aorfluid? gas. movement.

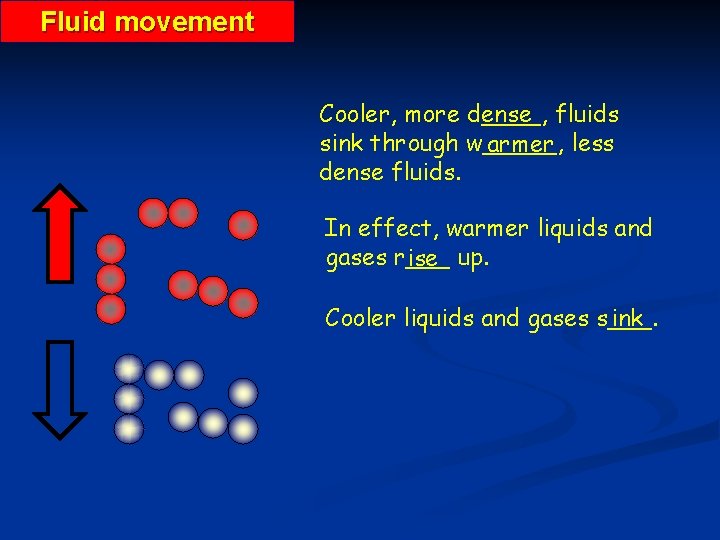
Fluid movement Cooler, more d____, ense fluids sink through w_____, armer less dense fluids. In effect, warmer liquids and gases r___ ise up. Cooler liquids and gases s___. ink

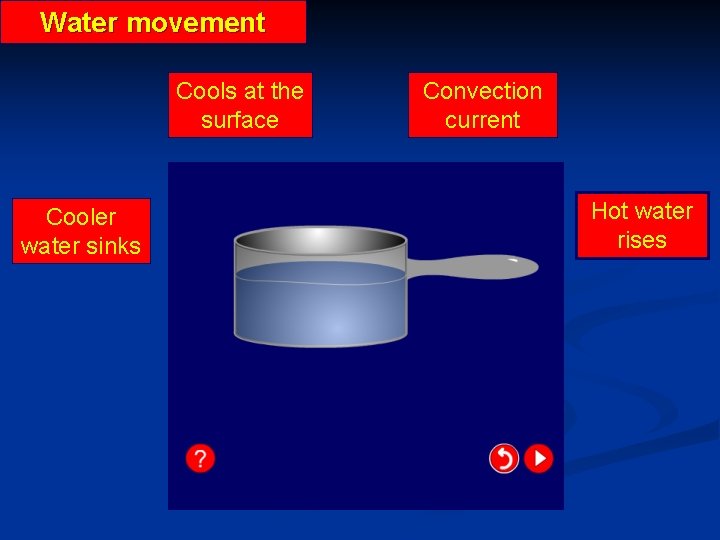
Water movement Cools at the surface Cooler water sinks Convection current Hot water rises

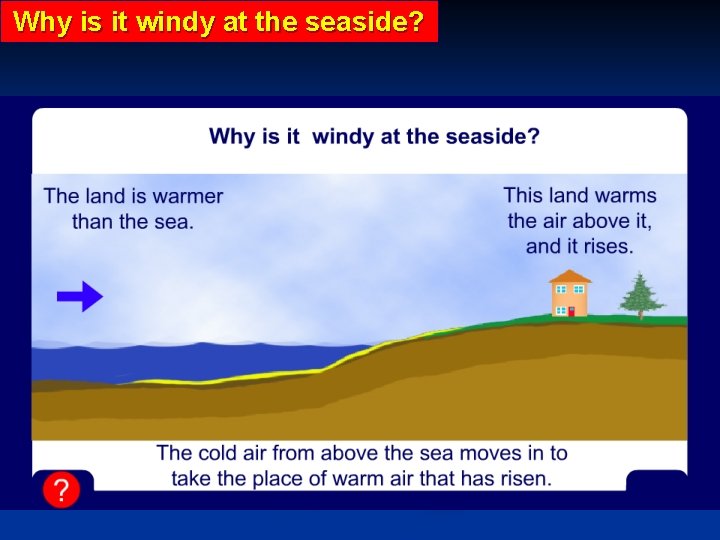
Why is it windy at the seaside?

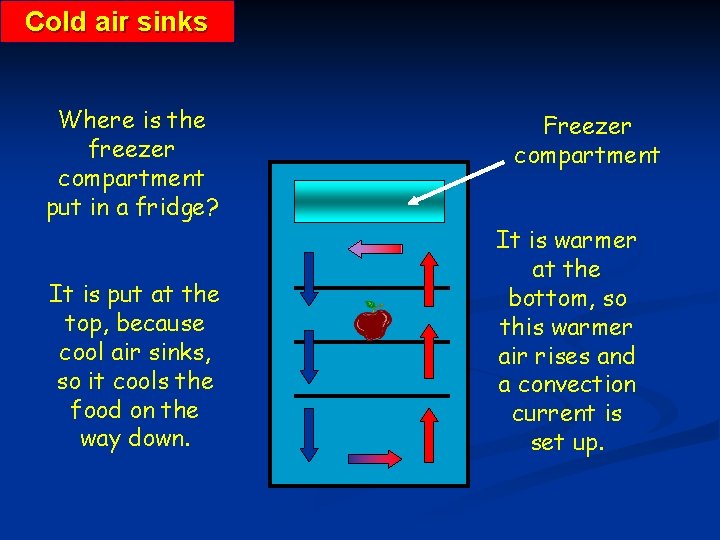
Cold air sinks Where is the freezer compartment put in a fridge? It is put at the top, because cool air sinks, so it cools the food on the way down. Freezer compartment It is warmer at the bottom, so this warmer air rises and a convection current is set up.

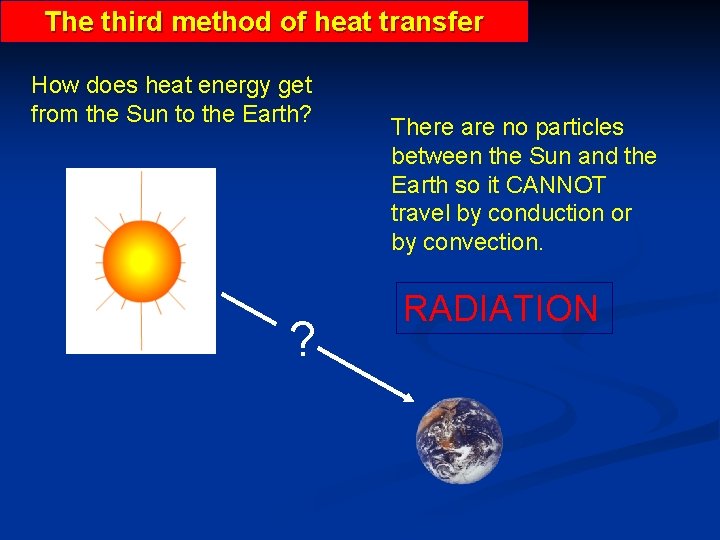
The third method of heat transfer How does heat energy get from the Sun to the Earth? ? There are no particles between the Sun and the Earth so it CANNOT travel by conduction or by convection. RADIATION

Emission experiment Four containers were filled with warm water. Which container would have the warmest water after ten minutes? Dull metal Shiny black Dull black shiny metal container would be the warmest after ten The _____ radiation back minutes because its shiny surface reflects heat _______ dull black container into the container so less is lost. The ____ emitting heat would be the coolest because it is the best at _______ radiation.

Absorption experiment Four containers were placed equidistant from a heater. Which container would have the warmest water after ten minutes? Dull metal Shiny black Dull black dull black container would be the warmest after ten The _____ radiation the best. minutes because its surface absorbs heat _______ shiny metal container would be the coolest because it is The _____ the poorest at _____ absorbing heat radiation.

Convection questions Why does hot air rise and cold air sink? Cool air is more dense than warm air, so the cool air ‘falls through’ the warm air. Why are boilers placed beneath hot water tanks in people’s homes? Hot water rises. So when the boiler heats the water, and the hot water rises, the water tank is filled with hot water.

Radiation questions Why are houses painted white in hot countries? White reflects heat radiation and keeps the house cooler. Why are shiny foil blankets wrapped around marathon runners at the end of a race? The shiny metal reflects the heat radiation from the runner back in, this stops the runner getting cold.

1. Which of the following is not a method of heat transfer? A. Radiation B. Insulation C. Conduction D. Convection

2. In which of the following are the particles closest together? A. Solid B. Liquid C. Gas D. Fluid

3. How does heat energy reach the Earth from the Sun? A. Radiation B. Conduction C. Convection D. Insulation

4. Which is the best surface for reflecting heat radiation? A. Shiny white B. Dull white C. Shiny black D. Dull black

5. Which is the best surface for absorbing heat radiation? A. Shiny white B. Dull white C. Shiny black D. Dull black

EFFECTS OF HEAT 1. 2. 3. 4. 5. Change in temperature Change in phase Change in size Chemical change Change in bodily functions of living organisms

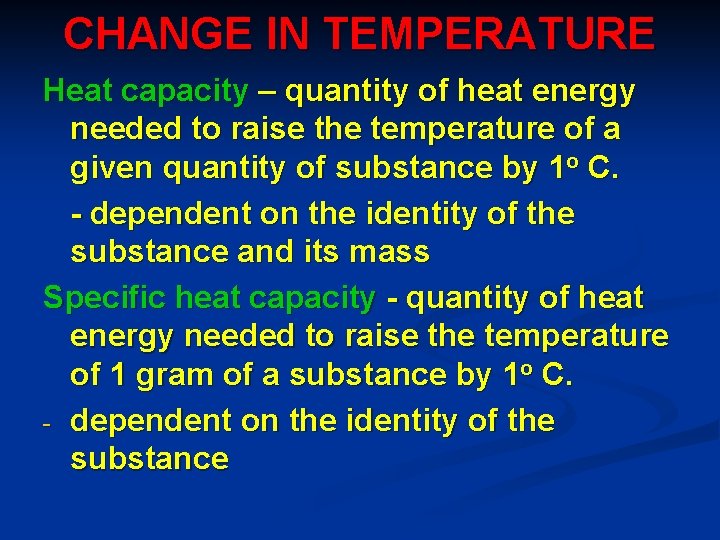
CHANGE IN TEMPERATURE Heat capacity – quantity of heat energy needed to raise the temperature of a given quantity of substance by 1 o C. - dependent on the identity of the substance and its mass Specific heat capacity - quantity of heat energy needed to raise the temperature of 1 gram of a substance by 1 o C. - dependent on the identity of the substance

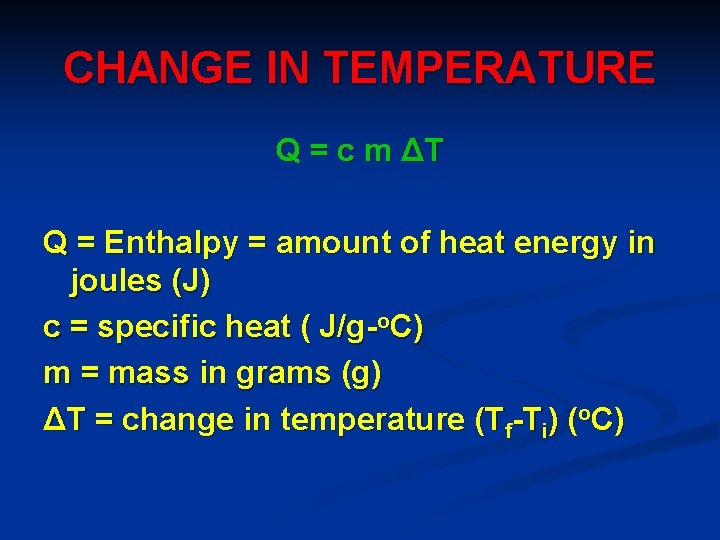
CHANGE IN TEMPERATURE Q = c m ΔT Q = Enthalpy = amount of heat energy in joules (J) c = specific heat ( J/g-o. C) m = mass in grams (g) ΔT = change in temperature (Tf-Ti) (o. C)

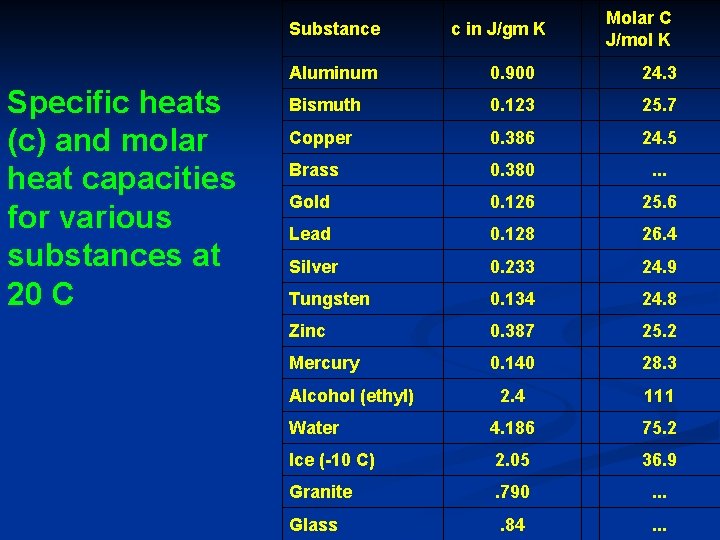
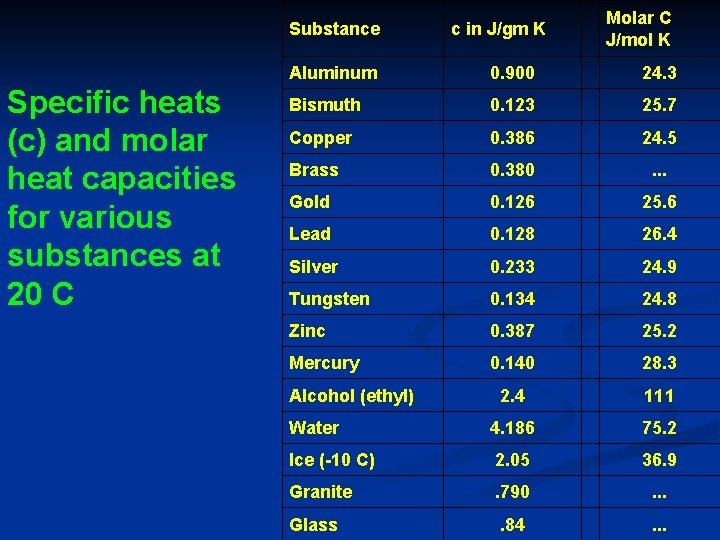
Substance Specific heats (c) and molar heat capacities for various substances at 20 C c in J/gm K Molar C J/mol K Aluminum 0. 900 24. 3 Bismuth 0. 123 25. 7 Copper 0. 386 24. 5 Brass 0. 380 . . . Gold 0. 126 25. 6 Lead 0. 128 26. 4 Silver 0. 233 24. 9 Tungsten 0. 134 24. 8 Zinc 0. 387 25. 2 Mercury 0. 140 28. 3 2. 4 111 Water 4. 186 75. 2 Ice (-10 C) 2. 05 36. 9 Granite . 790 . . . Glass . 84 . . . Alcohol (ethyl)

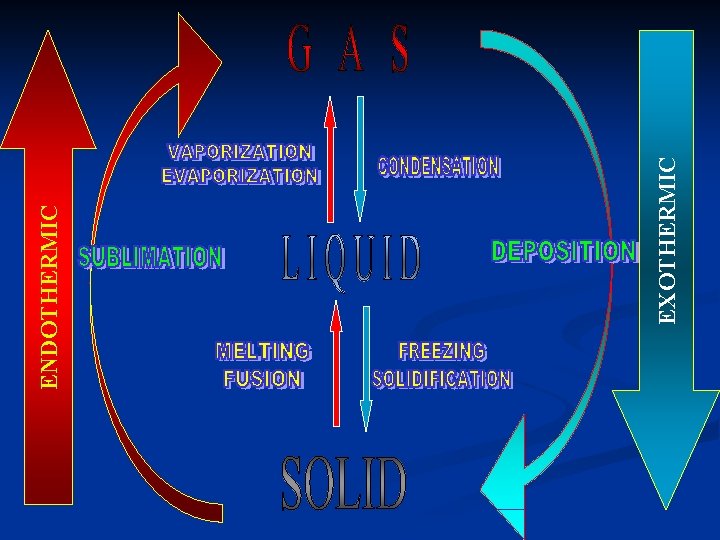
Heat gain, heat loss Endothermic change – system absorbs heat Exothermic change – system releases heat

EXOTHERMIC ENDOTHERMIC

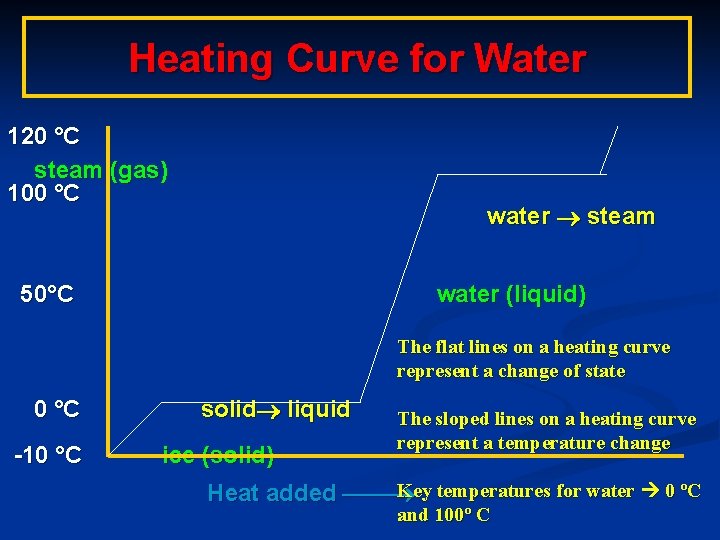
Heating Curve for Water 120 °C steam (gas) 100 °C water steam 50°C water (liquid) The flat lines on a heating curve represent a change of state 0 °C -10 °C solid liquid ice (solid) The sloped lines on a heating curve represent a temperature change Key temperatures for water 0 ºC Heat added and 100º C

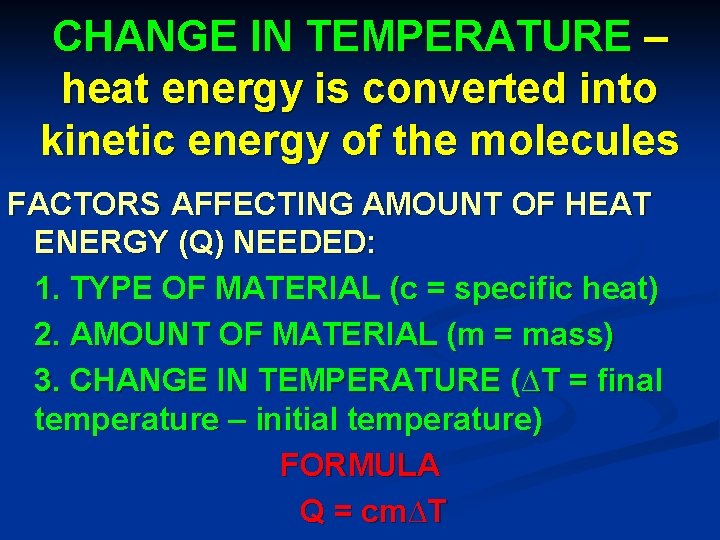
CHANGE IN TEMPERATURE – heat energy is converted into kinetic energy of the molecules FACTORS AFFECTING AMOUNT OF HEAT ENERGY (Q) NEEDED: 1. TYPE OF MATERIAL (c = specific heat) 2. AMOUNT OF MATERIAL (m = mass) 3. CHANGE IN TEMPERATURE (∆T = final temperature – initial temperature) FORMULA Q = cm∆T

Substance Specific heats (c) and molar heat capacities for various substances at 20 C c in J/gm K Molar C J/mol K Aluminum 0. 900 24. 3 Bismuth 0. 123 25. 7 Copper 0. 386 24. 5 Brass 0. 380 . . . Gold 0. 126 25. 6 Lead 0. 128 26. 4 Silver 0. 233 24. 9 Tungsten 0. 134 24. 8 Zinc 0. 387 25. 2 Mercury 0. 140 28. 3 2. 4 111 Water 4. 186 75. 2 Ice (-10 C) 2. 05 36. 9 Granite . 790 . . . Glass . 84 . . . Alcohol (ethyl)

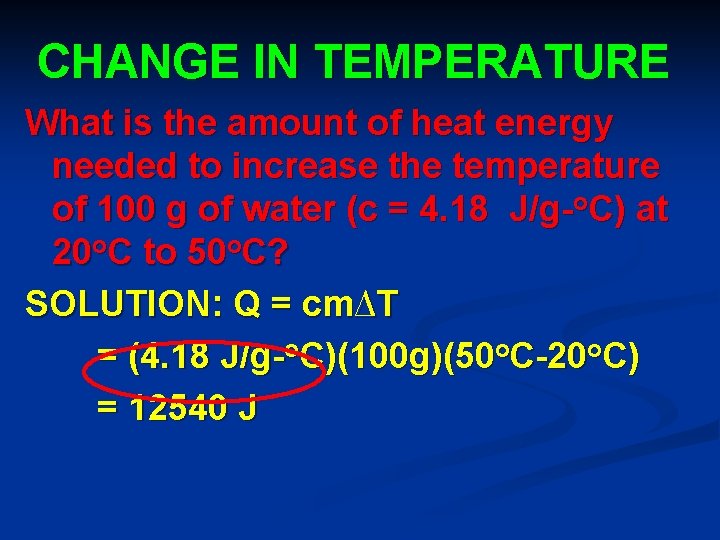
CHANGE IN TEMPERATURE What is the amount of heat energy needed to increase the temperature of 100 g of water (c = 4. 18 J/g-o. C) at 20 o. C to 50 o. C? SOLUTION: Q = cm∆T = (4. 18 J/g-o. C)(100 g)(50 o. C-20 o. C) = 12540 J

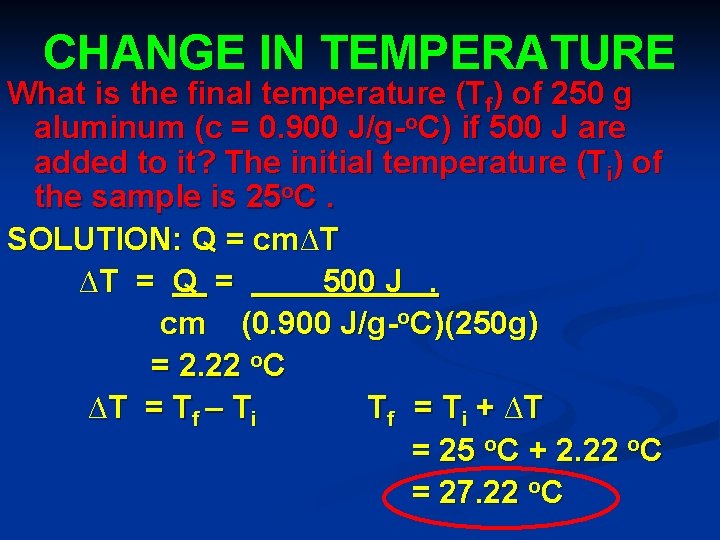
CHANGE IN TEMPERATURE What is the final temperature (Tf) of 250 g aluminum (c = 0. 900 J/g-o. C) if 500 J are added to it? The initial temperature (Ti) of the sample is 25 o. C. SOLUTION: Q = cm∆T ∆T = Q = 500 J. cm (0. 900 J/g-o. C)(250 g) = 2. 22 o. C ∆T = T f – T i Tf = T i + ∆T = 25 o. C + 2. 22 o. C = 27. 22 o. C

CHANGE IN PHASE – occurred whenever the supplied heat energy is enough to overcome the intermolecular forces of attraction - occurred at specific temperatures (freezing/melting temperatures) and (boiling/condensation temperatures)

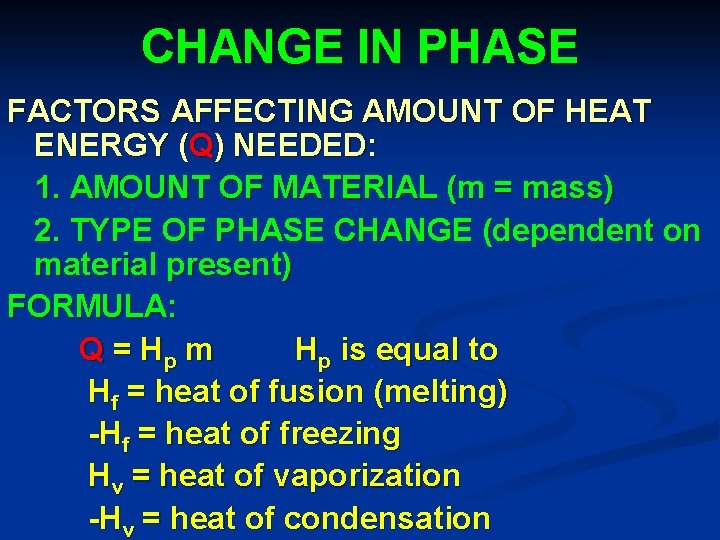
CHANGE IN PHASE FACTORS AFFECTING AMOUNT OF HEAT ENERGY (Q) NEEDED: 1. AMOUNT OF MATERIAL (m = mass) 2. TYPE OF PHASE CHANGE (dependent on material present) FORMULA: Q = Hp m Hp is equal to Hf = heat of fusion (melting) -Hf = heat of freezing Hv = heat of vaporization -Hv = heat of condensation

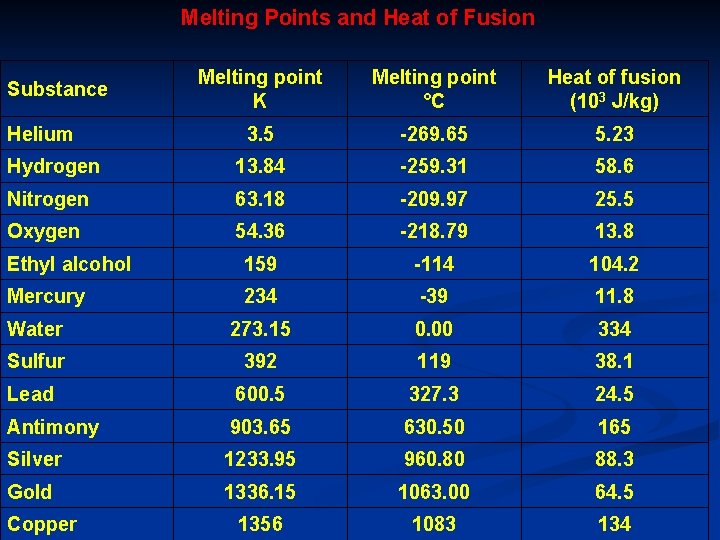
Melting Points and Heat of Fusion Melting point K Melting point °C Heat of fusion (103 J/kg) 3. 5 -269. 65 5. 23 Hydrogen 13. 84 -259. 31 58. 6 Nitrogen 63. 18 -209. 97 25. 5 Oxygen 54. 36 -218. 79 13. 8 Ethyl alcohol 159 -114 104. 2 Mercury 234 -39 11. 8 Water 273. 15 0. 00 334 Sulfur 392 119 38. 1 Lead 600. 5 327. 3 24. 5 Antimony 903. 65 630. 50 165 Silver 1233. 95 960. 80 88. 3 Gold 1336. 15 1063. 00 64. 5 1356 1083 134 Substance Helium Copper

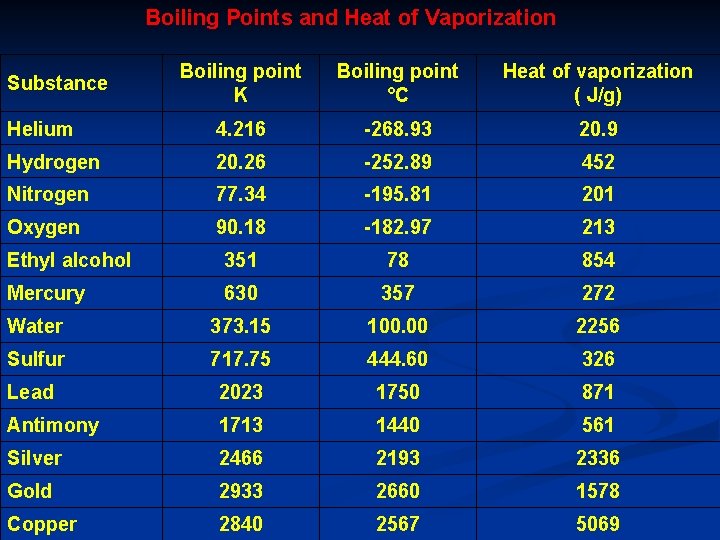
Boiling Points and Heat of Vaporization Boiling point K Boiling point °C Heat of vaporization ( J/g) Helium 4. 216 -268. 93 20. 9 Hydrogen 20. 26 -252. 89 452 Nitrogen 77. 34 -195. 81 201 Oxygen 90. 18 -182. 97 213 Ethyl alcohol 351 78 854 Mercury 630 357 272 Water 373. 15 100. 00 2256 Sulfur 717. 75 444. 60 326 Lead 2023 1750 871 Antimony 1713 1440 561 Silver 2466 2193 2336 Gold 2933 2660 1578 Copper 2840 2567 5069 Substance

CHANGE IN PHASE What is the amount of heat energy needed to change 200 g of water at 0 o. C to ice? (Hf ice = 334 J/g) SOLUTION: Q = Hf m = (-334 J/g) (200 g) = -66800 J Negative sign indicates exothermic reaction