Solutions Notes Words to Know Solution homogenous mixture






![Ion-product constant – Kw refers to the ionization of water [ ] = concentration Ion-product constant – Kw refers to the ionization of water [ ] = concentration](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-26.jpg)
![Example: Calculate [H+] or [OH-] as required for each of the following solutions at Example: Calculate [H+] or [OH-] as required for each of the following solutions at](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-27.jpg)
![p. H scale – because the [H+] in an aqueous solution is typically small, p. H scale – because the [H+] in an aqueous solution is typically small,](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-28.jpg)
![Example – Calculate the p. H or p. OH a. [H+] = 5. 9 Example – Calculate the p. H or p. OH a. [H+] = 5. 9](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-30.jpg)
![Since Kw = [H+][OH-] = 1. 0 x 10 -14 , p. H + Since Kw = [H+][OH-] = 1. 0 x 10 -14 , p. H +](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-31.jpg)
![In order to calculate the concentration from the p. H or p. OH, [H+] In order to calculate the concentration from the p. H or p. OH, [H+]](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-32.jpg)





- Slides: 50

Solutions Notes

Words to Know • Solution – homogenous mixture • Solvent – substance present in the largest amount • Solutes – substance present in the smallest amount • Aqueous solution – solutions with water as the solvent • Concentration – the amount of solute in a given volume of solution • Concentrated – large amount of solute dissolved in solvent • Dilute – small amount of solute dissolved in solvent

• Saturated – a solution that contains as much solute as will dissolve at that temperature • Unsaturated – a solution that hasn’t reached that limit of solute that will dissolve • Supersaturated - a solution that contains more solute than should dissolve at that temperature

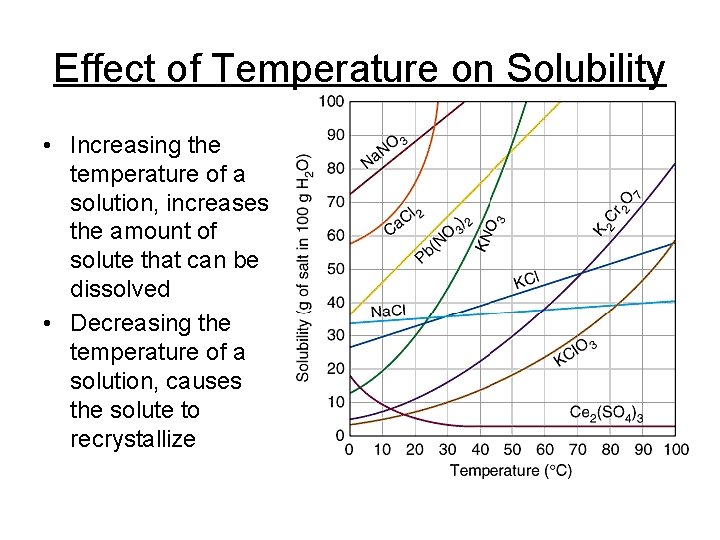
Effect of Temperature on Solubility • Increasing the temperature of a solution, increases the amount of solute that can be dissolved • Decreasing the temperature of a solution, causes the solute to recrystallize

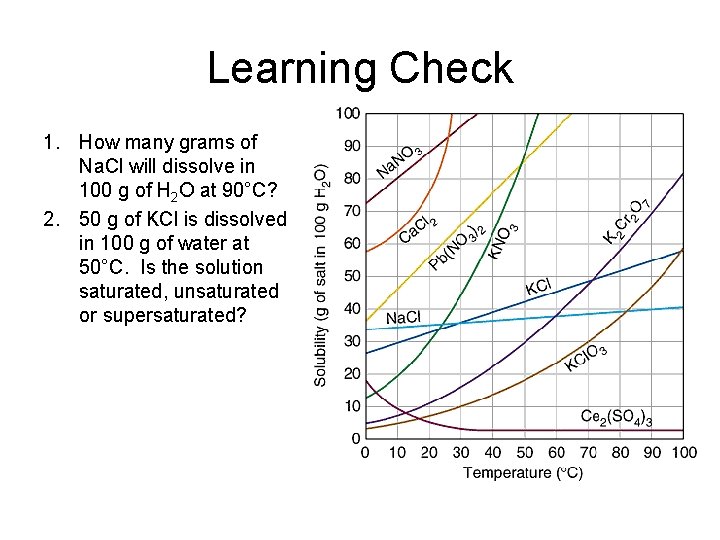
Learning Check 1. How many grams of Na. Cl will dissolve in 100 g of H 2 O at 90°C? 2. 50 g of KCl is dissolved in 100 g of water at 50°C. Is the solution saturated, unsaturated or supersaturated?

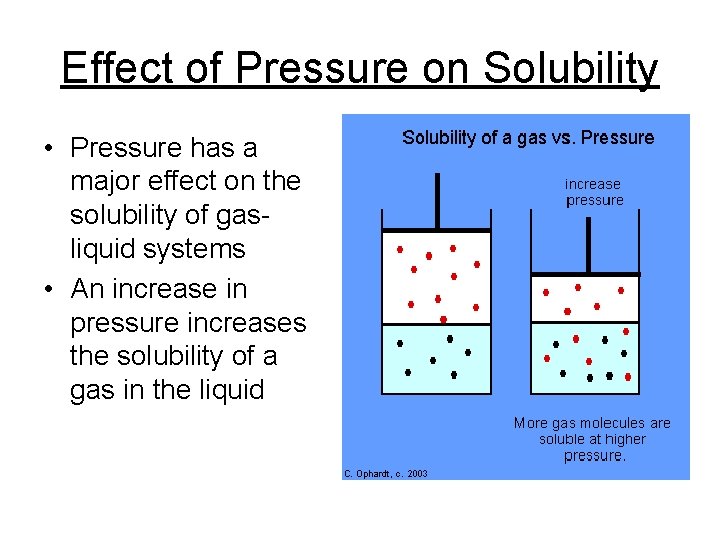
Effect of Pressure on Solubility • Pressure has a major effect on the solubility of gasliquid systems • An increase in pressure increases the solubility of a gas in the liquid

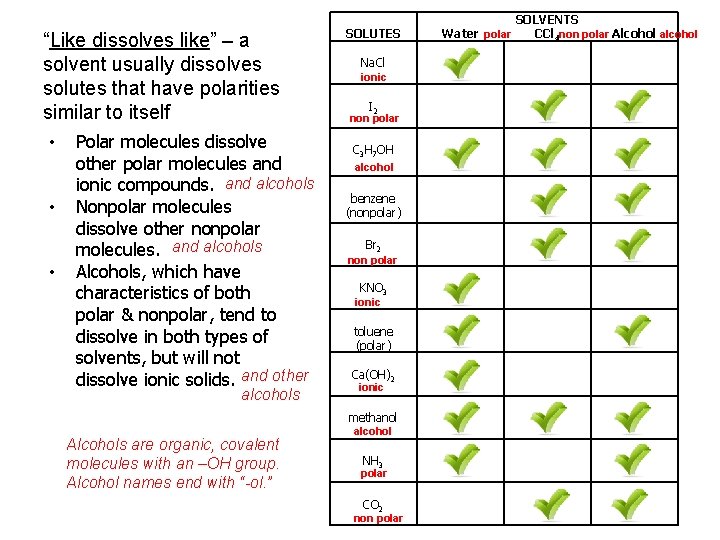
“Like dissolves like” – a solvent usually dissolves solutes that have polarities similar to itself • • • Polar molecules dissolve other polar molecules and ionic compounds. and alcohols Nonpolar molecules dissolve other nonpolar molecules. and alcohols Alcohols, which have characteristics of both polar & nonpolar, tend to dissolve in both types of solvents, but will not dissolve ionic solids. and other alcohols Alcohols are organic, covalent molecules with an –OH group. Alcohol names end with “-ol. ” SOLUTES Na. Cl ionic I 2 non polar C 3 H 7 OH alcohol benzene (nonpolar) Br 2 non polar KNO 3 ionic toluene (polar) Ca(OH)2 ionic methanol alcohol NH 3 polar CO 2 non polar SOLVENTS Water polar CCl 4 non polar Alcohol alcohol

Colligative properties - the physical changes that result from adding solute to a solvent. Colligative Properties depend on how many solute particles are present as well as the solvent amount, but they do NOT depend on the type of solute particles. • • • Boiling Point Elevation Freezing Point Depression Osmotic Pressure Increasing Vapor Pressure Lowering Conductivity Increasing More particles/ions = greater change

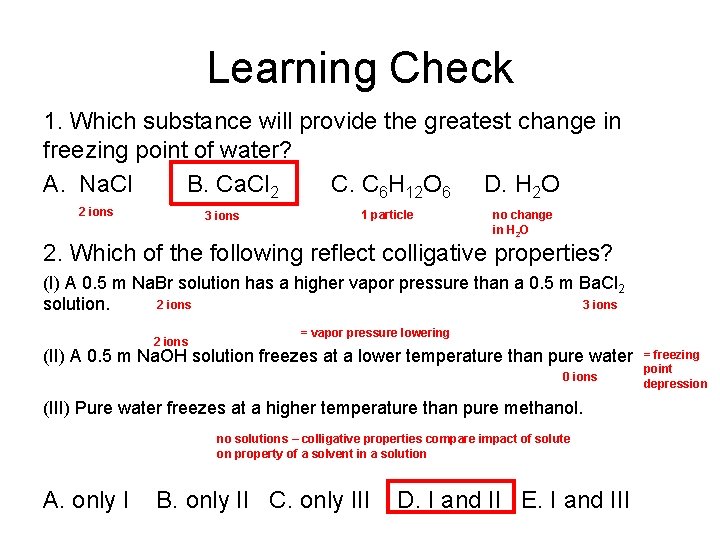
Learning Check 1. Which substance will provide the greatest change in freezing point of water? A. Na. Cl B. Ca. Cl 2 C. C 6 H 12 O 6 D. H 2 O 2 ions 3 ions 1 particle no change in H 2 O 2. Which of the following reflect colligative properties? (I) A 0. 5 m Na. Br solution has a higher vapor pressure than a 0. 5 m Ba. Cl 2 3 ions 2 ions solution. = vapor pressure lowering 2 ions (II) A 0. 5 m Na. OH solution freezes at a lower temperature than pure water 0 ions (III) Pure water freezes at a higher temperature than pure methanol. no solutions – colligative properties compare impact of solute on property of a solvent in a solution A. only I B. only II C. only III D. I and II E. I and III = freezing point depression

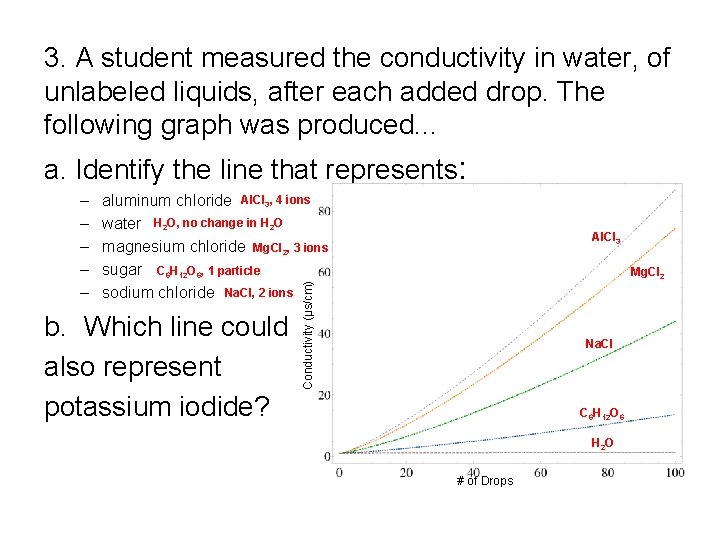
3. A student measured the conductivity in water, of unlabeled liquids, after each added drop. The following graph was produced. . . a. Identify the line that represents: aluminum chloride Al. Cl 3, 4 ions water H 2 O, no change in H 2 O magnesium chloride Mg. Cl 2, 3 ions sugar C 6 H 12 O 6, 1 particle sodium chloride Na. Cl, 2 ions b. Which line could also represent potassium iodide? Al. Cl 3 Mg. Cl 2 Conductivity (µs/cm) – – – Na. Cl C 6 H 12 O 6 H 2 O # of Drops

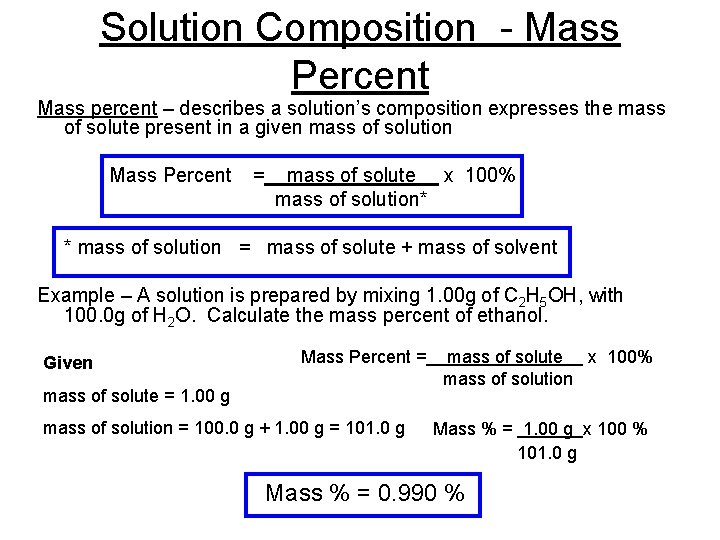
Solution Composition - Mass Percent Mass percent – describes a solution’s composition expresses the mass of solute present in a given mass of solution Mass Percent = mass of solute x 100% mass of solution* * mass of solution = mass of solute + mass of solvent Example – A solution is prepared by mixing 1. 00 g of C 2 H 5 OH, with 100. 0 g of H 2 O. Calculate the mass percent of ethanol. Given mass of solute = 1. 00 g Mass Percent = mass of solute x 100% mass of solution = 100. 0 g + 1. 00 g = 101. 0 g Mass % = 1. 00 g x 100 % 101. 0 g Mass % = 0. 990 %

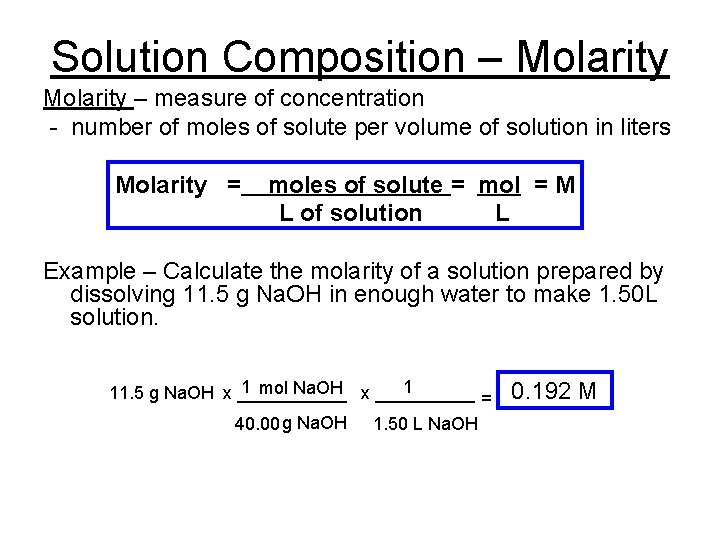
Solution Composition – Molarity – measure of concentration - number of moles of solute per volume of solution in liters Molarity = moles of solute = mol = M L of solution L Example – Calculate the molarity of a solution prepared by dissolving 11. 5 g Na. OH in enough water to make 1. 50 L solution. 1 mol Na. OH x _____ 1 11. 5 g Na. OH x ______ = 40. 00 g Na. OH 1. 50 L Na. OH 0. 192 M

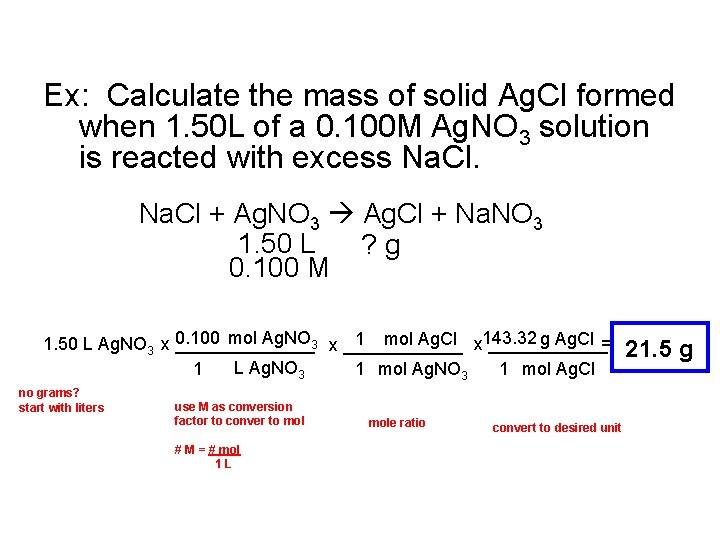
Ex: Calculate the mass of solid Ag. Cl formed when 1. 50 L of a 0. 100 M Ag. NO 3 solution is reacted with excess Na. Cl + Ag. NO 3 Ag. Cl + Na. NO 3 1. 50 L ? g 0. 100 M 0. 100 mol Ag. NO 3 x ______ 143. 32 g Ag. Cl = 1 mol Ag. Cl x ______ 1. 50 L Ag. NO 3 x _______ 1 L Ag. NO 3 1 mol Ag. Cl no grams? start with liters use M as conversion factor to conver to mol # M = # mol 1 L mole ratio convert to desired unit 21. 5 g

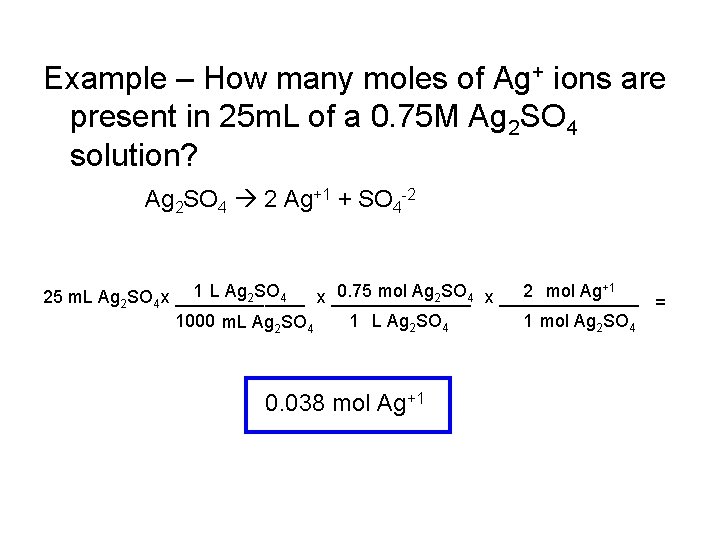
Example – How many moles of Ag+ ions are present in 25 m. L of a 0. 75 M Ag 2 SO 4 solution? Ag 2 SO 4 2 Ag+1 + SO 4 -2 1 L Ag 2 SO 4 x _______ 0. 75 mol Ag 2 SO 4 x _______ 2 mol Ag+1 25 m. L Ag 2 SO x _______ 4 = 1000 m. L Ag 2 SO 4 1 mol Ag 2 SO 4 0. 038 mol Ag+1

Learning check Calculate the molarity of a solution prepared by dissolving 25. 6 g Na. C 2 H 3 O 2 in enough water to make 200. 0 m. L solution.

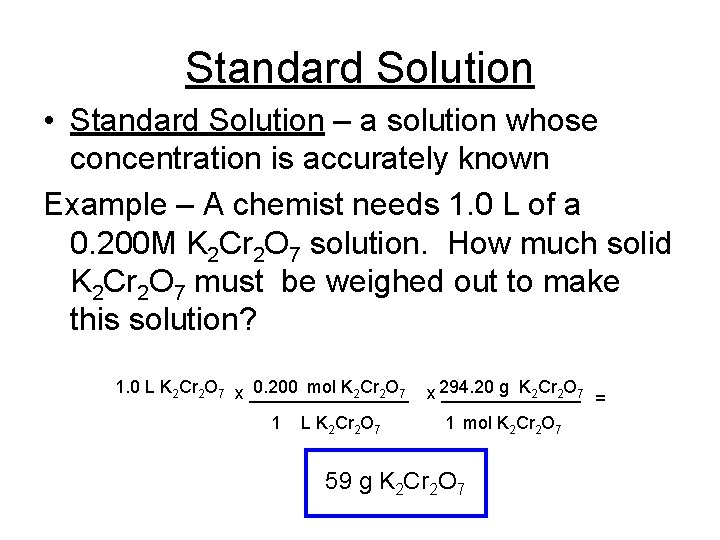
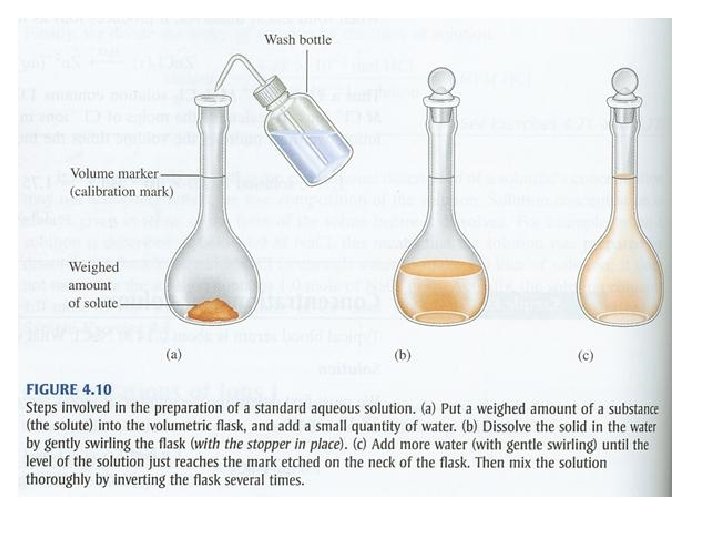
Standard Solution • Standard Solution – a solution whose concentration is accurately known Example – A chemist needs 1. 0 L of a 0. 200 M K 2 Cr 2 O 7 solution. How much solid K 2 Cr 2 O 7 must be weighed out to make this solution? 1. 0 L K 2 Cr 2 O 7 x ________ 0. 200 mol K 2 Cr 2 O 7 x _______ 294. 20 g K 2 Cr 2 O 7 = 1 L K 2 Cr 2 O 7 1 mol K 2 Cr 2 O 7 59 g K 2 Cr 2 O 7


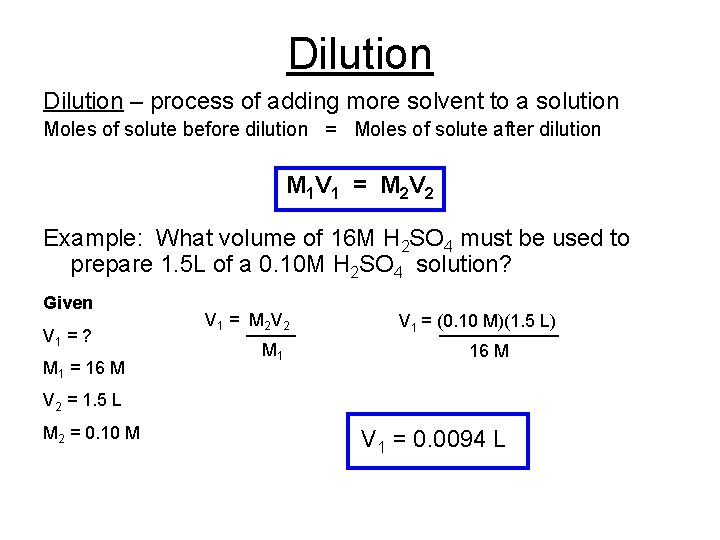
Dilution – process of adding more solvent to a solution Moles of solute before dilution = Moles of solute after dilution M 1 V 1 = M 2 V 2 Example: What volume of 16 M H 2 SO 4 must be used to prepare 1. 5 L of a 0. 10 M H 2 SO 4 solution? Given V 1 = ? M 1 = 16 M V 1 = M 2 V 2 _____ M 1 V 1 = (0. 10 M)(1. 5 L) ______ 16 M V 2 = 1. 5 L M 2 = 0. 10 M V 1 = 0. 0094 L

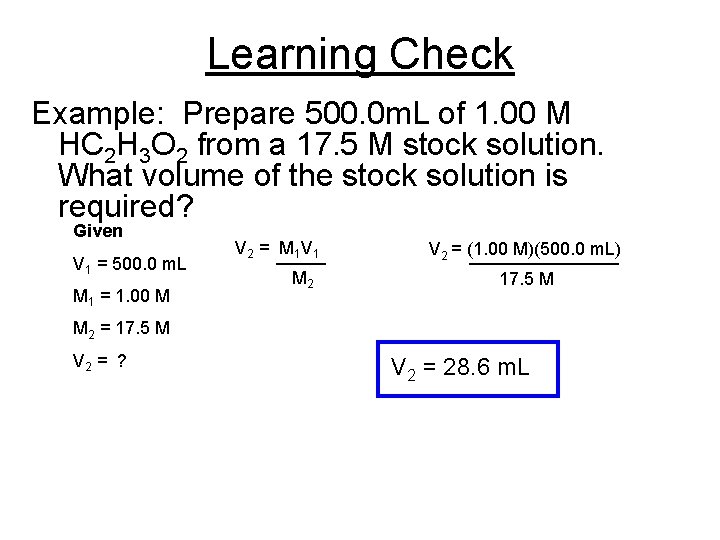
Learning Check Example: Prepare 500. 0 m. L of 1. 00 M HC 2 H 3 O 2 from a 17. 5 M stock solution. What volume of the stock solution is required? Given V 1 = 500. 0 m. L M 1 = 1. 00 M V 2 = M 1 V 1 _____ M 2 V 2 = (1. 00 M)(500. 0 m. L) ________ 17. 5 M M 2 = 17. 5 M V 2 = ? V 2 = 28. 6 m. L

Notes. Acids and Bases

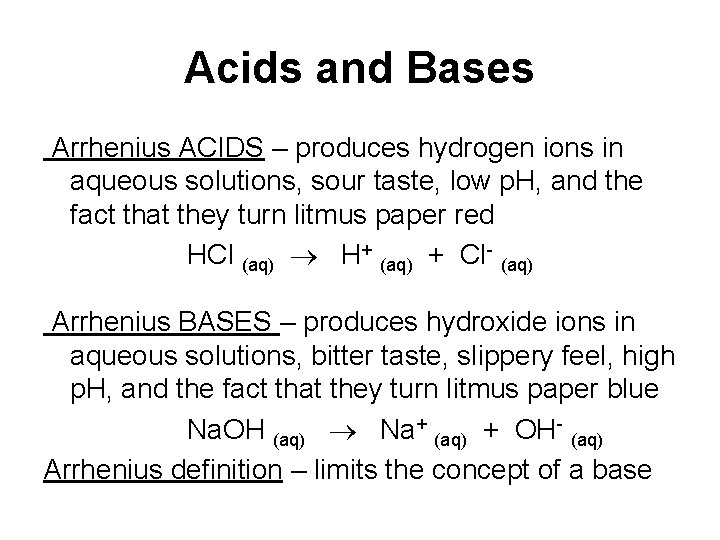
Acids and Bases Arrhenius ACIDS – produces hydrogen ions in aqueous solutions, sour taste, low p. H, and the fact that they turn litmus paper red HCl (aq) H+ (aq) + Cl- (aq) Arrhenius BASES – produces hydroxide ions in aqueous solutions, bitter taste, slippery feel, high p. H, and the fact that they turn litmus paper blue Na. OH (aq) Na+ (aq) + OH- (aq) Arrhenius definition – limits the concept of a base

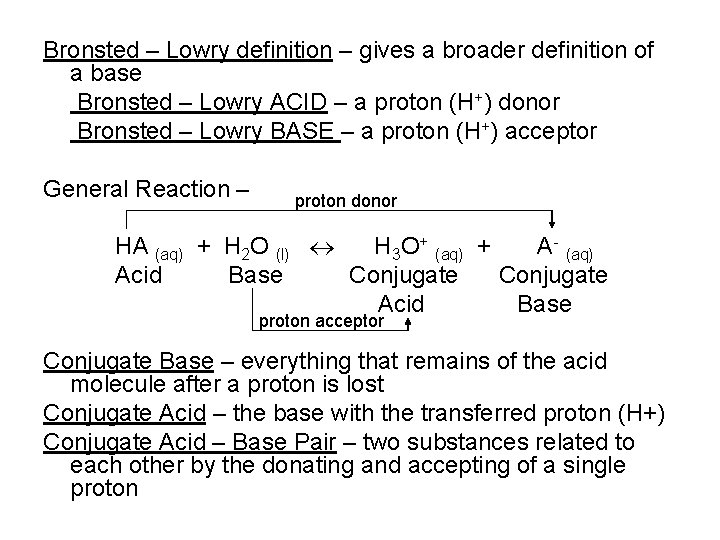
Bronsted – Lowry definition – gives a broader definition of a base Bronsted – Lowry ACID – a proton (H+) donor Bronsted – Lowry BASE – a proton (H+) acceptor General Reaction – proton donor HA (aq) + H 2 O (l) H 3 O+ (aq) + A- (aq) Acid Base Conjugate Acid Base proton acceptor Conjugate Base – everything that remains of the acid molecule after a proton is lost Conjugate Acid – the base with the transferred proton (H+) Conjugate Acid – Base Pair – two substances related to each other by the donating and accepting of a single proton

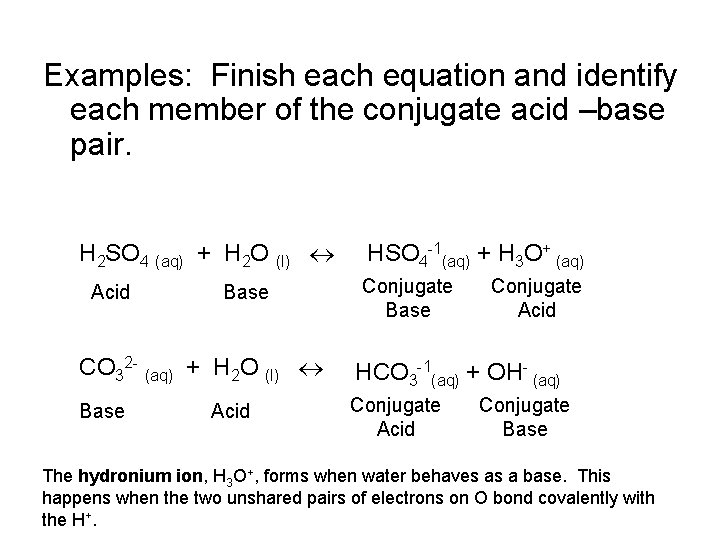
Examples: Finish each equation and identify each member of the conjugate acid –base pair. H 2 SO 4 (aq) + H 2 O (l) Acid Base HSO 4 -1(aq) + H 3 O+ (aq) Conjugate Base Conjugate Acid CO 32 - (aq) + H 2 O (l) HCO 3 -1(aq) + OH- (aq) Base Conjugate Acid Conjugate Base The hydronium ion, H 3 O+, forms when water behaves as a base. This happens when the two unshared pairs of electrons on O bond covalently with the H+.

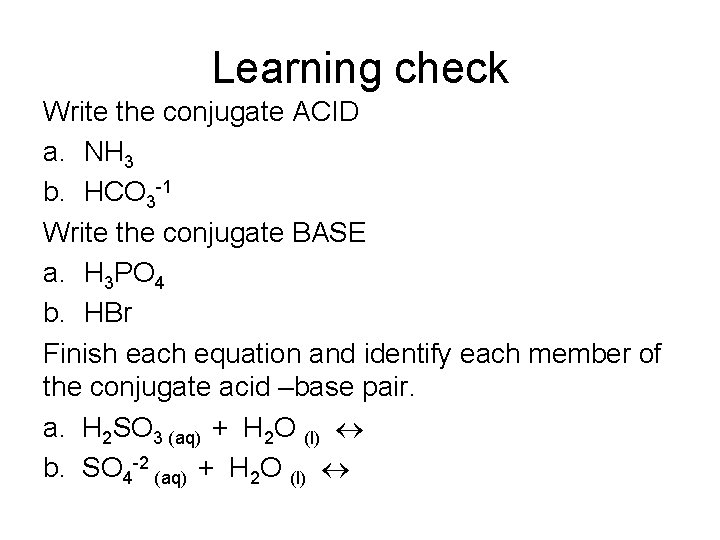
Learning check Write the conjugate ACID a. NH 3 b. HCO 3 -1 Write the conjugate BASE a. H 3 PO 4 b. HBr Finish each equation and identify each member of the conjugate acid –base pair. a. H 2 SO 3 (aq) + H 2 O (l) b. SO 4 -2 (aq) + H 2 O (l)

Water as an Acid and a Base Amphoteric – a substance that can behave as either an acid or a base - water is the most common amphoteric substance Ionization of Water – H 2 O (l) + H 2 O (l) H 3 O+ (aq) + OH- (aq) In the shorthand form: H 2 O (l) H+ (aq) + OH- (aq)
![Ionproduct constant Kw refers to the ionization of water concentration Ion-product constant – Kw refers to the ionization of water [ ] = concentration](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-26.jpg)
Ion-product constant – Kw refers to the ionization of water [ ] = concentration Kw = [H+][OH-] [H+] = hydrogen ion concentration in M [OH-] = hydroxide ion concentration in M At 25 C, Kw = [H+][OH-] = [1. 0 x 10 -7] = 1. 0 x 10 -14 If [H+] increases, the [OH-] decreases, so the products of the two is still 1. 0 x 10 -14. There are three possible situations – 1. A neutral solution, where [H+] = [OH-] 2. An acidic solution, where [H+] [OH-] 3. A basic solution, where [H+] [OH-]
![Example Calculate H or OH as required for each of the following solutions at Example: Calculate [H+] or [OH-] as required for each of the following solutions at](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-27.jpg)
Example: Calculate [H+] or [OH-] as required for each of the following solutions at 25 C, for each solution state whether it is neutral, acidic, or basic. a. 1. 0 x 10 -5 M OHKw = [H+][OH-] 1 x 10 -14 = [H+][1. 0 x 10 -5 M] [H+] = 1. 0 x 10 -9 M BASIC b. 10. 0 M H+ Kw = [H+][OH-] 1 x 10 -14 = [10. 0 M][OH-] = 1. 00 x 10 -15 M ACIDIC
![p H scale because the H in an aqueous solution is typically small p. H scale – because the [H+] in an aqueous solution is typically small,](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-28.jpg)
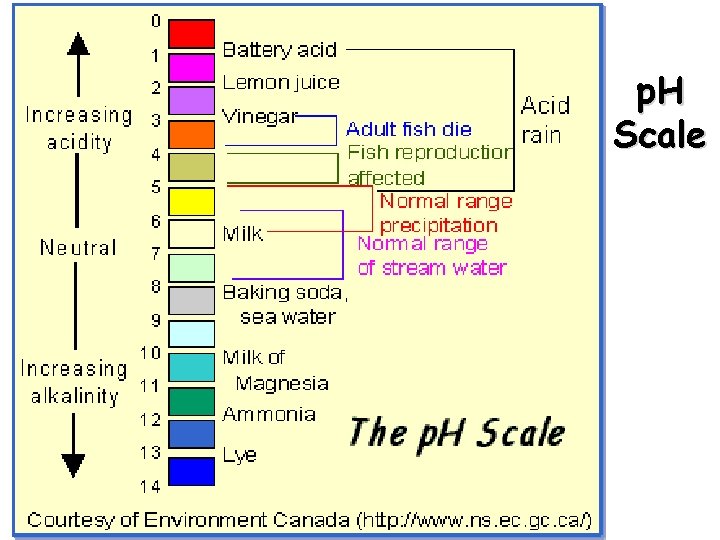
p. H scale – because the [H+] in an aqueous solution is typically small, logarithms are used to express solution acidity p. H = -log [H+] Graphing calculator 1. Press the +/- key 2. Press the log key 3. Enter the [H+] p. OH = -log [OH-] Non graphing calculator 1. Enter the [H+] 2. Press the log key 3. Press the +/- key Significant Figure Rule – The number of places to the right of the decimal for a log must be equal to the number of significant figures in the original number.

p. H Scale
![Example Calculate the p H or p OH a H 5 9 Example – Calculate the p. H or p. OH a. [H+] = 5. 9](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-30.jpg)
Example – Calculate the p. H or p. OH a. [H+] = 5. 9 x 10 -9 M b. [OH-] = 2. 4 x 10 -6 M p. H = - log [H+] p. H = - log (5. 9 x 10 -9 M) p. H = 8. 23 p. OH = - log [OH-] p. OH = - log (2. 4 x 10 -6 M) p. OH = 5. 62
![Since Kw HOH 1 0 x 10 14 p H Since Kw = [H+][OH-] = 1. 0 x 10 -14 , p. H +](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-31.jpg)
Since Kw = [H+][OH-] = 1. 0 x 10 -14 , p. H + p. OH = 14. 00 Example - The p. H of blood is about 7. 4. What is the p. OH of blood? p. H + p. OH =14. 00 7. 4 + p. OH = 14. 00 p. OH = 6. 6
![In order to calculate the concentration from the p H or p OH H In order to calculate the concentration from the p. H or p. OH, [H+]](https://slidetodoc.com/presentation_image_h/af9869d62aa99567f52e700a047d08f4/image-32.jpg)
In order to calculate the concentration from the p. H or p. OH, [H+] = 10 -p. H Graphing calculator 1. Press the 2 nd function, then log 2. Press the +/- key 3. Enter the p. H [OH-] = 10 -p. OH Non-graphing calculator 1. Enter the p. H 2. Press the +/- key 3. Press the inverse log key

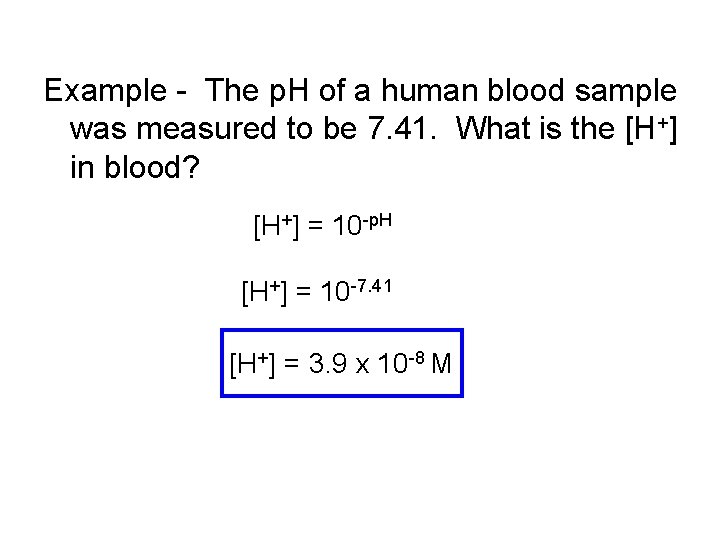
Example - The p. H of a human blood sample was measured to be 7. 41. What is the [H+] in blood? [H+] = 10 -p. H [H+] = 10 -7. 41 [H+] = 3. 9 x 10 -8 M

Example – The p. OH of the water in a fish tank is found to be 6. 59. What is the [H+] for this water? [OH-] = 10 -p. OH [OH-] = 10 -6. 59 [OH-] = 2. 6 x 10 -7 M Kw = [H+][OH-] 1 x 10 -14 = [H+][2. 6 x 10 -7 M] [H+] = 3. 8 x 10 -8 M

Learning check • Determine the p. H of a solution with a hydrogen ion concentration of 3. 2 x 10 -12 M. • What is the [OH-] concentration of a solution with a hydrogen ion concentration of 8. 9 x 10 -4 M? • What is the p. H of a solution with a hydroxide ion concentration of 5. 7 x 10 -10 M?

How Do We Measure p. H? • For less accurate measurements, one can use – Litmus paper • “Red” paper turns blue above ~p. H = 8 • “Blue” paper turns red below ~p. H = 5 – An indicator

How Do We Measure p. H? For more accurate measurements, one uses a p. H meter, which measures the voltage in the solution.

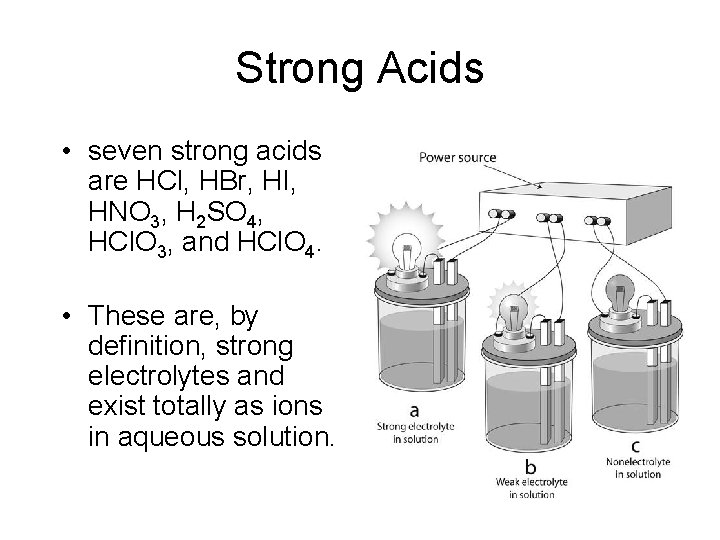
Strong Acids • seven strong acids are HCl, HBr, HI, HNO 3, H 2 SO 4, HCl. O 3, and HCl. O 4. • These are, by definition, strong electrolytes and exist totally as ions in aqueous solution.

Strong Bases • Strong bases are the soluble hydroxides, which are the alkali metal and heavier alkaline earth metal hydroxides (Ca 2+, Sr 2+, and Ba 2+). • Again, these substances dissociate completely in aqueous solution, strong electrolytes

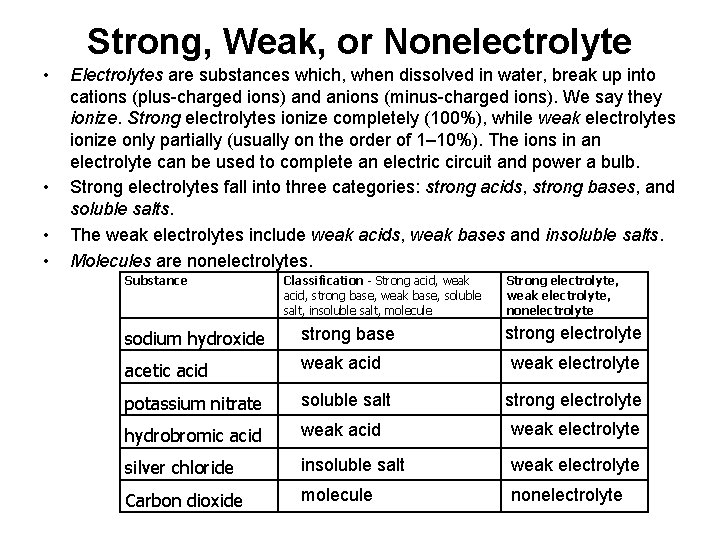
Strong, Weak, or Nonelectrolyte • • Electrolytes are substances which, when dissolved in water, break up into cations (plus-charged ions) and anions (minus-charged ions). We say they ionize. Strong electrolytes ionize completely (100%), while weak electrolytes ionize only partially (usually on the order of 1– 10%). The ions in an electrolyte can be used to complete an electric circuit and power a bulb. Strong electrolytes fall into three categories: strong acids, strong bases, and soluble salts. The weak electrolytes include weak acids, weak bases and insoluble salts. Molecules are nonelectrolytes. Substance Classification - Strong acid, weak acid, strong base, weak base, soluble salt, insoluble salt, molecule Strong electrolyte, weak electrolyte, nonelectrolyte sodium hydroxide strong base strong electrolyte acetic acid weak acid potassium nitrate soluble salt strong electrolyte hydrobromic acid weak electrolyte silver chloride insoluble salt weak electrolyte Carbon dioxide molecule nonelectrolyte weak electrolyte

Learning check Substance Classification - Strong electrolyte, acid, weak acid, strong base, weak electrolyte, weak base, soluble salt, nonelectrolyte insoluble salt, molecule chloric acid barium carbonate nitric acid sulfurous acid strontium sulfate ethanol octane (gasoline)

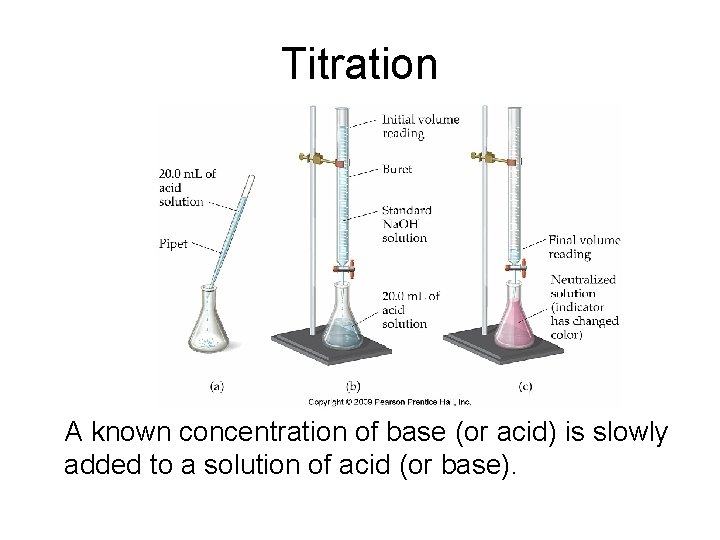
Titration A known concentration of base (or acid) is slowly added to a solution of acid (or base).

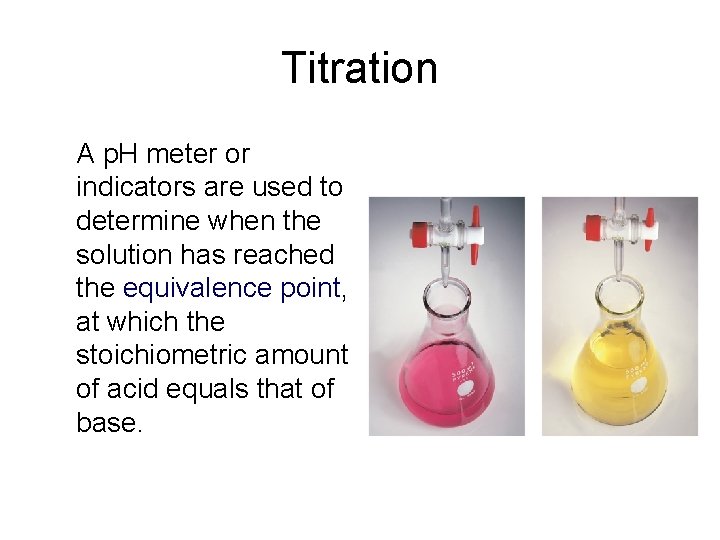
Titration A p. H meter or indicators are used to determine when the solution has reached the equivalence point, at which the stoichiometric amount of acid equals that of base.

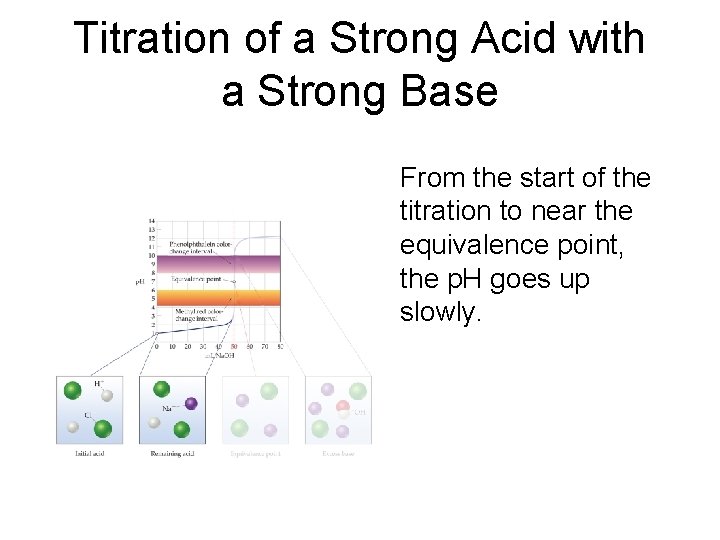
Titration of a Strong Acid with a Strong Base From the start of the titration to near the equivalence point, the p. H goes up slowly.

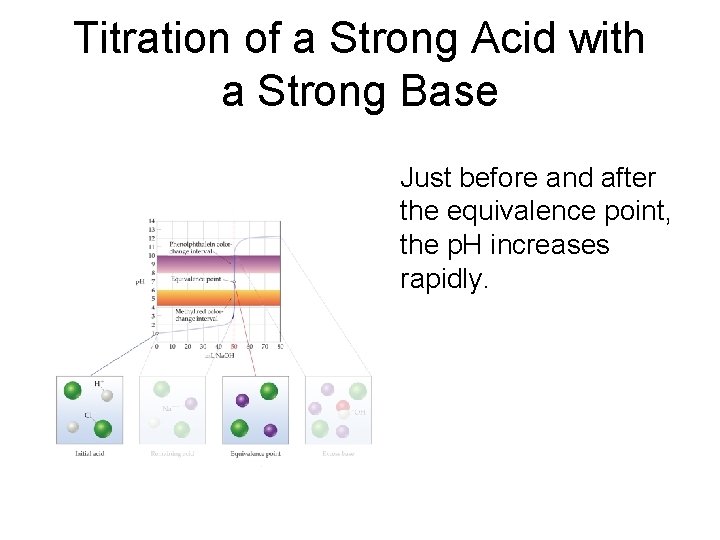
Titration of a Strong Acid with a Strong Base Just before and after the equivalence point, the p. H increases rapidly.

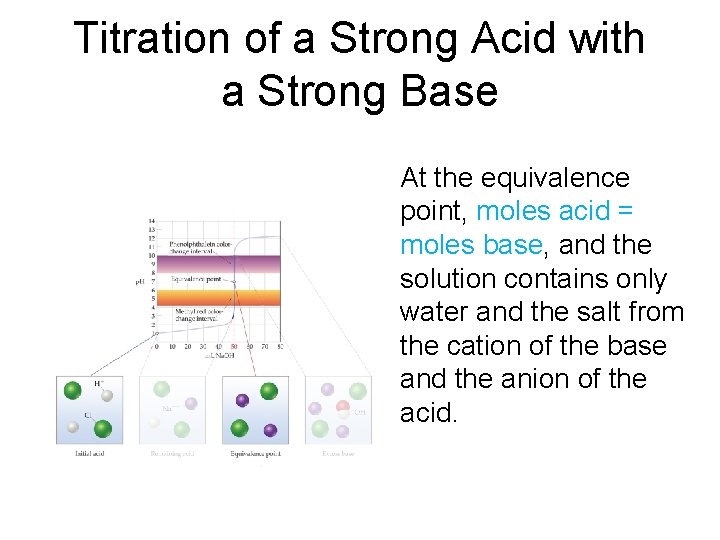
Titration of a Strong Acid with a Strong Base At the equivalence point, moles acid = moles base, and the solution contains only water and the salt from the cation of the base and the anion of the acid.

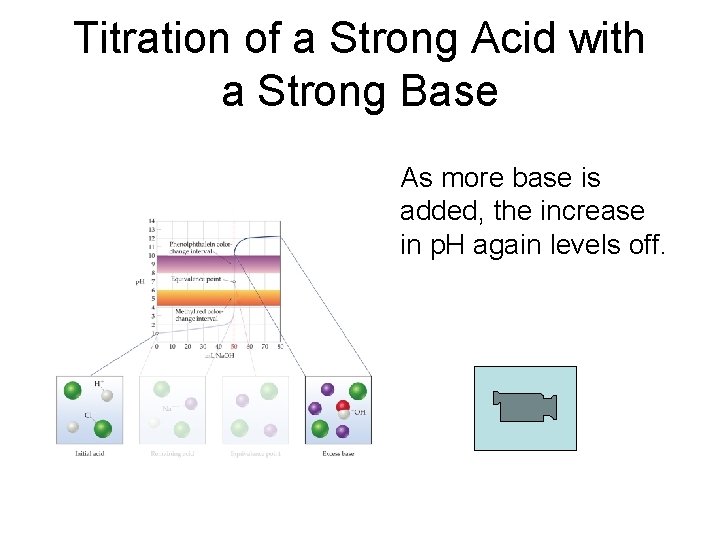
Titration of a Strong Acid with a Strong Base As more base is added, the increase in p. H again levels off.

Neutralization Reaction = Acid + Base Salt + Water Salt – ionic compound containing a positive ion other than H+ and a negative ion other than OH-

Buffered solutions – resists a change in its p. H even when a strong acid or base is added to it - A solution is buffered in the presence of a weak acid and its conjugate base

p. H and p. OH Calculations