SNC 2 D CHEMISTRY CHEMICAL REACTIONS Physical Chemical














- Slides: 22
SNC 2 D CHEMISTRY CHEMICAL REACTIONS � Physical & Chemical Changes (P. ~)

Physical & Chemical Changes Some of the most useful and powerful properties of matter are those related to how and why matter changes. There are countless changes in matter that affect us every day: for example, applying heat to an egg, burning gasoline, freezing water, and mixing oil and vinegar, to name a few. Observing, understanding and categorizing kinds of change are an important first step to making use of change. February 1, 2022 2 DCHEM - Physical & Chemical Changes 1

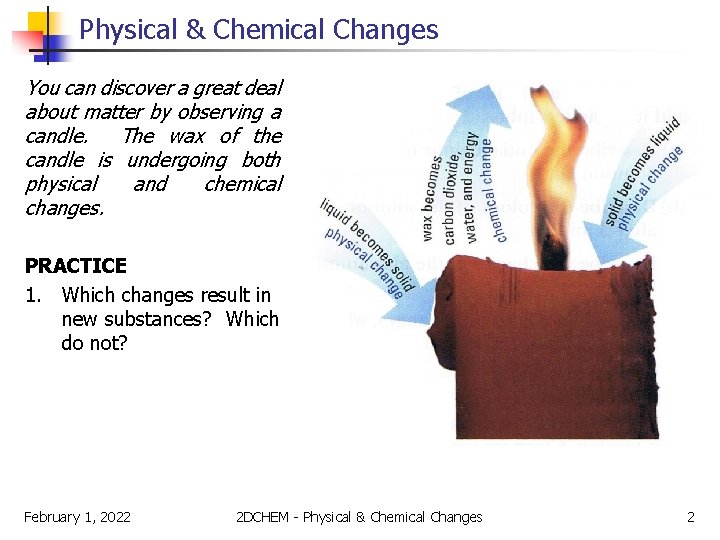
Physical & Chemical Changes You can discover a great deal about matter by observing a candle. The wax of the candle is undergoing both physical and chemical changes. PRACTICE 1. Which changes result in new substances? Which do not? February 1, 2022 2 DCHEM - Physical & Chemical Changes 2

Observations RECALL! An observation is information that you get through your senses. You observe that a rose is red and has a sweet smell. You may also note that it has sharp thorns on its stem. You may count the petals on the flower and the leaves on the stem and measure the length of the stem. February 1, 2022 2 DCHEM - Physical & Chemical Changes 3

Observations When people describe the qualities of objects and events, the observations are qualitative. The colour of the rose, the odour of the flower, and the sharpness of the thorns are all qualitative observations. QUALITATIVE DATA v information that you get through your senses February 1, 2022 2 DCHEM - Physical & Chemical Changes 4

Observations based on measurements or counting are quantitative since they deal with the quantities of things. The length of a rose’s stem, the number of petals, and the number of leaves are quantitative observations. QUANTITATIVE DATA v information based on measurements or counting February 1, 2022 2 DCHEM - Physical & Chemical Changes 5

Observations NOTE! The evidence gathered in an experiment is often described as the ultimate authority in science. With this in mind you must gather and communicate your qualitative and quantitative evidence clearly and accurately. Although the kind of observations are often dictated by the question asked at the beginning of the investigation, both qualitative and quantitative evidence is often gathered. Do not make the mistake of forgetting to record qualitative evidence when you are gathering quantitative evidence. February 1, 2022 2 DCHEM - Physical & Chemical Changes 6

Physical Changes of state - melting, freezing, evaporation, condensation, sublimation, deposition - are physical changes. Dissolving is also a physical change. When you dissolve sugar in water, the sugar particles spread out, but they are still there, as sugar particles. You can reverse the process by evaporating the water and collecting the sugar. NOTE! Most physical changes are easy to reverse. February 1, 2022 2 DCHEM - Physical & Chemical Changes 7

Physical Change PHYSICAL CHANGE v substance remains the same but changes state or form v chemical properties do not change v generally easy to reverse v dissolving, melting, … February 1, 2022 2 DCHEM - Physical & Chemical Changes 8

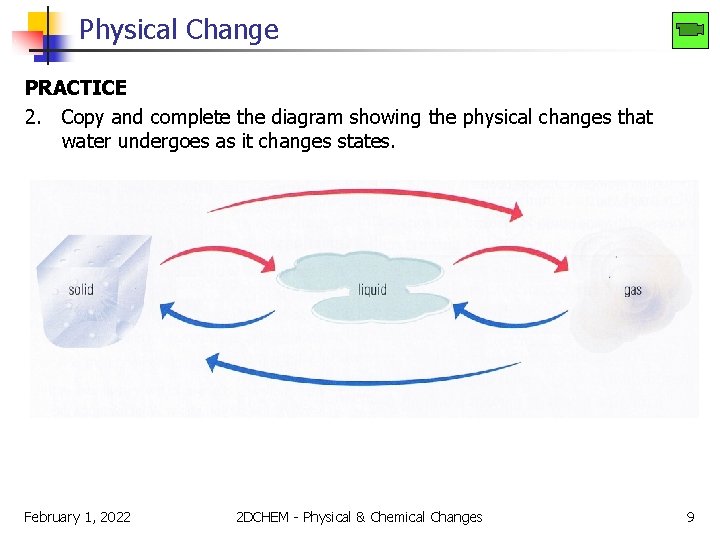
Physical Change PRACTICE 2. Copy and complete the diagram showing the physical changes that water undergoes as it changes states. February 1, 2022 2 DCHEM - Physical & Chemical Changes 9

Chemical Change Burning, cooking, and rusting are all examples of chemical changes. A chemical change always involves the formation of a new substance. For example, if you have ever mixed vinegar and baking soda you have experienced a chemical change. The bubbles that form are carbon dioxide gas – a new substance. February 1, 2022 2 DCHEM - Physical & Chemical Changes 10

Chemical Change CHEMICAL CHANGE v produces new substances different chemical properties v breaks compounds into atoms v burning, cooking, rusting, . . . February 1, 2022 with 2 DCHEM - Physical & Chemical Changes 11

Chemical Change NOTE! While some chemical changes are easy to categorize others are not so easy. So how to do you distinguish between the two? While there are clues that can help you decide whether a chemical or physical change has occurred it is important that you do not come to a conclusion too quickly. All of these clues suggest that a new substance has been produced, but any one of them could also accompany a physical change. You must consider several clues in order to determine what type of change has taken place. February 1, 2022 2 DCHEM - Physical & Chemical Changes 12

Chemical Change PRACTICE 3. There are 6 distinctive clues (either alone or together) that indicate a chemical change has occurred. What are they? CHEMICAL CHANGE CLUES � a new colour appears � a new odour appears � heat/light is given off/absorbed � bubbles of gas are formed � a solid material (precipitate) forms � the change is difficult to reverse February 1, 2022 2 DCHEM - Physical & Chemical Changes 13

Physical & Chemical Changes – DYK? Many chemical changes, such as those that occur when something burns, cannot be reversed. However, some chemical changes can be reversed. When you recharge a battery, you use electricity to reverse chemical changes that took place inside the battery to supply power. PHYSICAL & CHEMICAL CHANGES v some are reversible, while others are not February 1, 2022 2 DCHEM - Physical & Chemical Changes 14

Exothermic & Endothermic Reactions The explosion of dynamite, the freezing of water, and the burning of wood are all processes in which thermal energy (heat) is given out to the surroundings. Physical and chemical changes that release heat to the surroundings are said to be exothermic. For example, consider the combustion of gasoline: gasoline + oxygen � carbon dioxide + water + heat February 1, 2022 2 DCHEM - Physical & Chemical Changes 15

Exothermic & Endothermic Reactions EXOTHERMIC REACTION v chemical reaction that releases (energy) to the surroundings February 1, 2022 heat 2 DCHEM - Physical & Chemical Changes 16

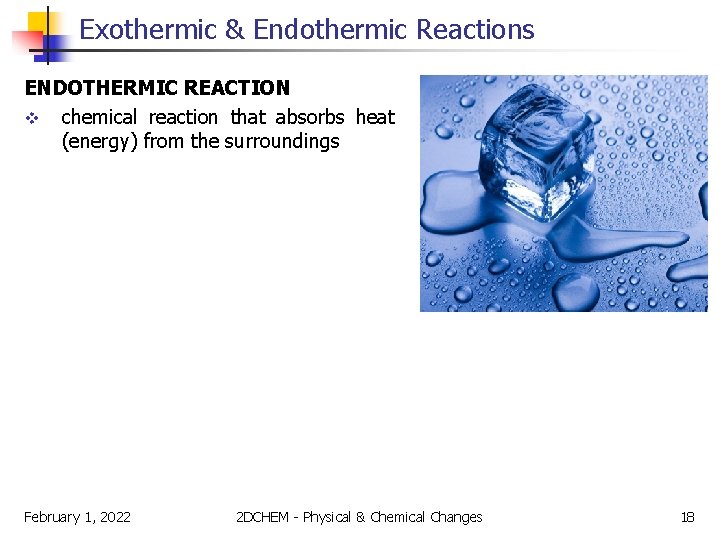
Exothermic & Endothermic Reactions On the other hand, other physical and chemical changes take place only when energy is continuously supplied to the reactants. Reactions that absorb heat from the surroundings are said to be endothermic. For example, consider the melting of ice: ice + heat �water February 1, 2022 2 DCHEM - Physical & Chemical Changes 17

Exothermic & Endothermic Reactions ENDOTHERMIC REACTION v chemical reaction that absorbs heat (energy) from the surroundings February 1, 2022 2 DCHEM - Physical & Chemical Changes 18

�Check Your Learning 1. Classify each of the following as a physical or chemical change. (a) Dry ice, solid carbon dioxide, sublimes at room temperature. (b) Salt dissolves in water. (c) Iron rusts in a damp environment. (d) Gasoline burns in the presence of oxygen. (e) A dead organism slowly decomposes. February 1, 2022 2 DCHEM - Physical & Chemical Changes P P C C C 19

�Check Your Learning 2. When sodium carbonate is added to water, the sodium carbonate dissolves. When hydrochloric acid is added to the solution, the solution fizzes. What kinds of changes have occurred? Explain. dissolves = change of state = physical fizzes = bubbles of gas are formed = chemical February 1, 2022 2 DCHEM - Physical & Chemical Changes 20

Activity: The Mint-Cola Fountain BACKGROUND The mint-cola fountain experiment may be one of the most popular science novelties of all time. It begins by dropping mints into a bottle of carbonated cola drink. Within a few seconds, a giant fountain of cola comes whooshing out of the bottle. QUESTION How does it work? (3 reasons) • • • carbon dioxide (dissolved in the cola) surface tension pits in the surface of the mint February 1, 2022 2 DCHEM - Physical & Chemical Changes 21
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Skeleton hand whmis
Skeleton hand whmis Sistema nervioso central
Sistema nervioso central Water density
Water density Snc lajaa venissieux
Snc lajaa venissieux Snc
Snc Síndrome de pfeiffer tipo 2
Síndrome de pfeiffer tipo 2 Snc database
Snc database Basic redox reactions
Basic redox reactions Types of reactions chemistry
Types of reactions chemistry 5 types of reactions chemistry
5 types of reactions chemistry Type of reactions chemistry
Type of reactions chemistry Methane oxygen reaction
Methane oxygen reaction Chemistry reactions
Chemistry reactions Stoichiometry island diagram
Stoichiometry island diagram