SNC 2 P CHEMISTRY CHEMICAL REACTIONS THEIR PRACTICAL



- Slides: 21

SNC 2 P CHEMISTRY CHEMICAL REACTIONS & THEIR PRACTICAL APPLICATIONS Ionic & Molecular Compounds (P. ~)

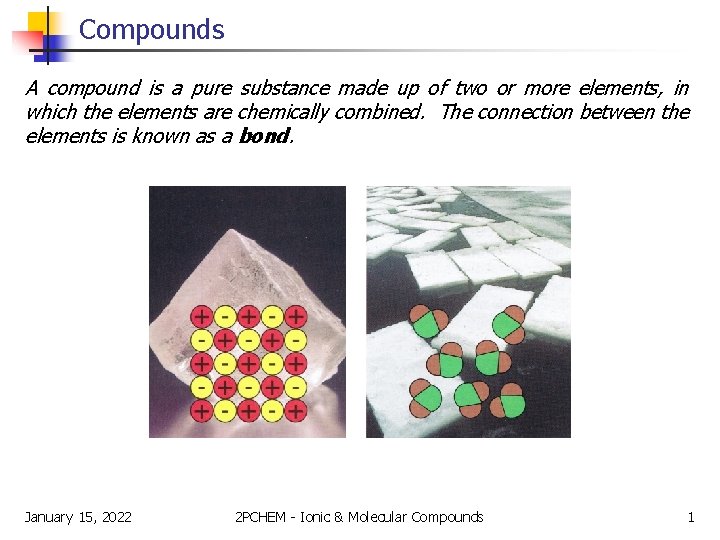
Compounds A compound is a pure substance made up of two or more elements, in which the elements are chemically combined. The connection between the elements is known as a bond. January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 1

Compounds NOTE! A small change in the way the atoms combine can make a big difference in the chemical and physical properties of compounds. January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 2

Compounds PRACTICE 1. What is the main difference between an element and a compound? an element is a pure substance made up of all the same atoms while a compound is a pure substance made up of two or more elements January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 3

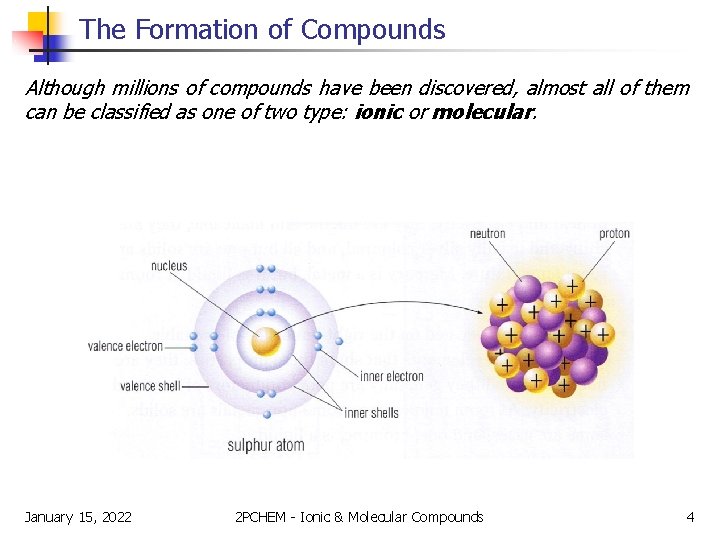
The Formation of Compounds Although millions of compounds have been discovered, almost all of them can be classified as one of two type: ionic or molecular. January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 4

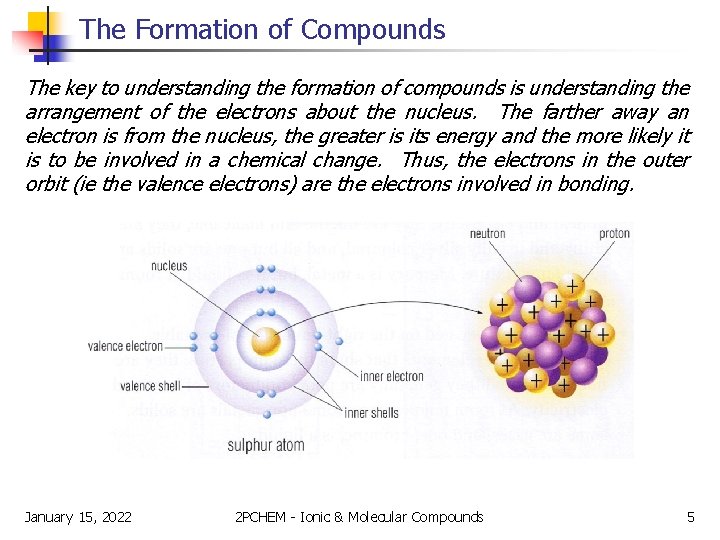
The Formation of Compounds The key to understanding the formation of compounds is understanding the arrangement of the electrons about the nucleus. The farther away an electron is from the nucleus, the greater is its energy and the more likely it is to be involved in a chemical change. Thus, the electrons in the outer orbit (ie the valence electrons) are the electrons involved in bonding. January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 5

The Formation of Compounds ELECTRONS ARE THE KEY!! Electrons can move from one orbit to another. The farther an electron is from the nucleus, the greater is its energy and the more likely it is to be involved in a chemical change. The electrons involved in bonding are located in the outer shell (i. e. the valence electrons). The number of electrons in the outer shell are determined by their location in the periodic table. When elements form compounds they either lose/gain/share electrons so that they have the same electron arrangement of the closest noble gas. Noble gases do not easily form compounds because their arrangements of electrons are particularly stable (i. e. their outer shell is full). January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 6

The Formation of Compounds ATOMS v are most stable when the outer orbit is full (2 or 8 e’s) v get a full outer orbit by: � losing e’s (becomes + ion) ionic bond � gaining e’s (becomes – ion) � sharing e’s covalent bond � He, Ne, … NOTE! In either case the ionic/molecular compound formed is electrically neutral. January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 7

Activity: Ionic Puzzle Piece (WS 2) INSTRUCTIONS A. Read the activity “ 2 PCHEM - WS 2 (Ionic Puzzle Piece Activity)”. B. Follow the instructions given (i. e. steps 1 to 6). C. Answer questions 1 to 7 on the back of the sheet (as per the instructions). NOTE! When you have completed the worksheet (and answered the questions) be sure to submit it to your teacher for evaluation. January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 8

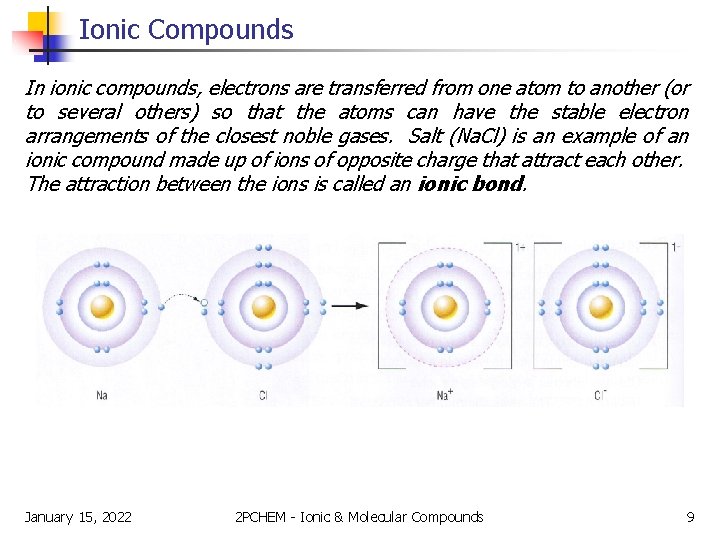
Ionic Compounds In ionic compounds, electrons are transferred from one atom to another (or to several others) so that the atoms can have the stable electron arrangements of the closest noble gases. Salt (Na. Cl) is an example of an ionic compound made up of ions of opposite charge that attract each other. The attraction between the ions is called an ionic bond. January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 9

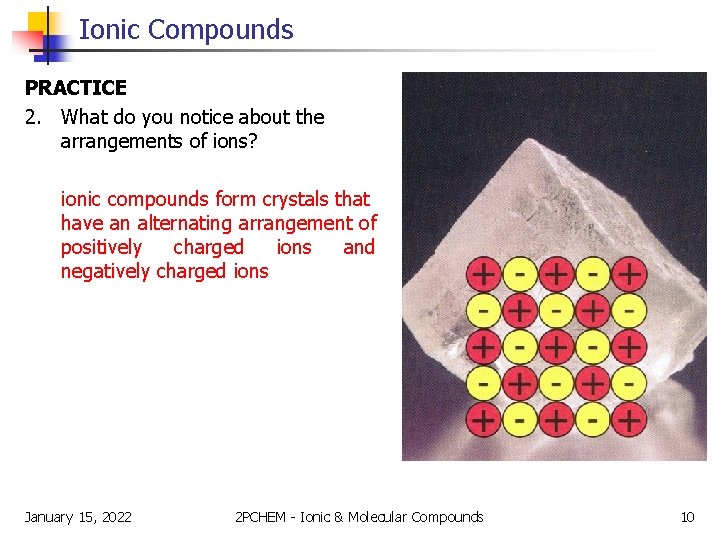
Ionic Compounds PRACTICE 2. What do you notice about the arrangements of ions? ionic compounds form crystals that have an alternating arrangement of positively charged ions and negatively charged ions January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 10

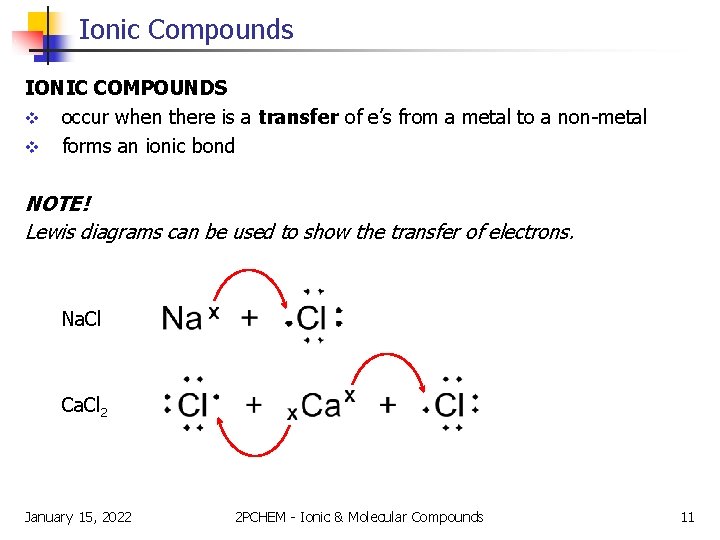
Ionic Compounds IONIC COMPOUNDS v occur when there is a transfer of e’s from a metal to a non-metal v forms an ionic bond NOTE! Lewis diagrams can be used to show the transfer of electrons. Na. Cl Ca. Cl 2 January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 11

Ionic Compounds Ionic compounds have a number of properties in common including hardness, melting points, solubility, and conductivity. PROPERTIES OF IONIC COMPOUNDS v are solids at room temperature v have high melting points (i. e. strong force of attraction between charged ions) v form solutions that conduct electricity January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 12

Ionic Compounds PRACTICE 3. following pairs of atoms: (a) K + F (KF) (b) Na + O (Na 2 O) (c) Mg + I (Mg. I 2) January 15, 2022 (d) Na + N (e) Be + S (f) Li + O 2 PCHEM - Ionic & Molecular Compounds (Na 3 N) (Be. S) (Li 2 O) 13

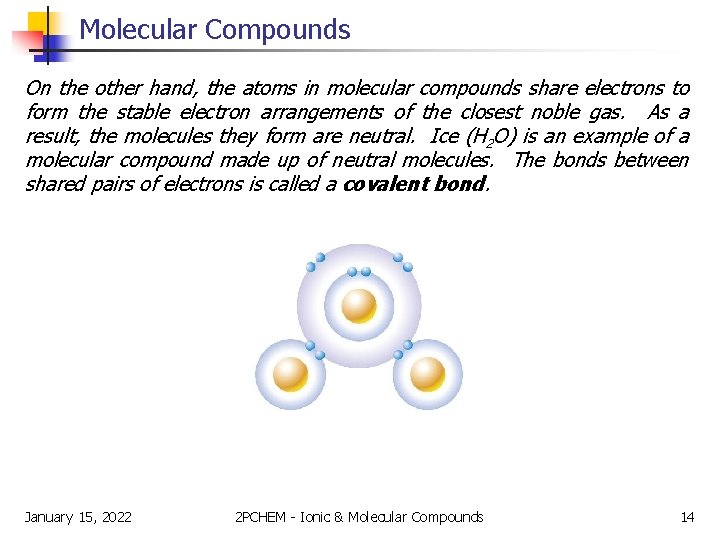
Molecular Compounds On the other hand, the atoms in molecular compounds share electrons to form the stable electron arrangements of the closest noble gas. As a result, the molecules they form are neutral. Ice (H 2 O) is an example of a molecular compound made up of neutral molecules. The bonds between shared pairs of electrons is called a covalent bond. January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 14

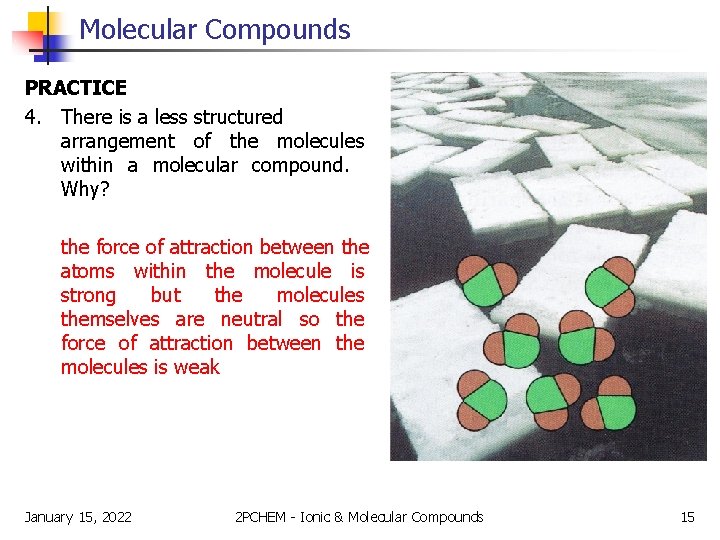
Molecular Compounds PRACTICE 4. There is a less structured arrangement of the molecules within a molecular compound. Why? the force of attraction between the atoms within the molecule is strong but the molecules themselves are neutral so the force of attraction between the molecules is weak January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 15

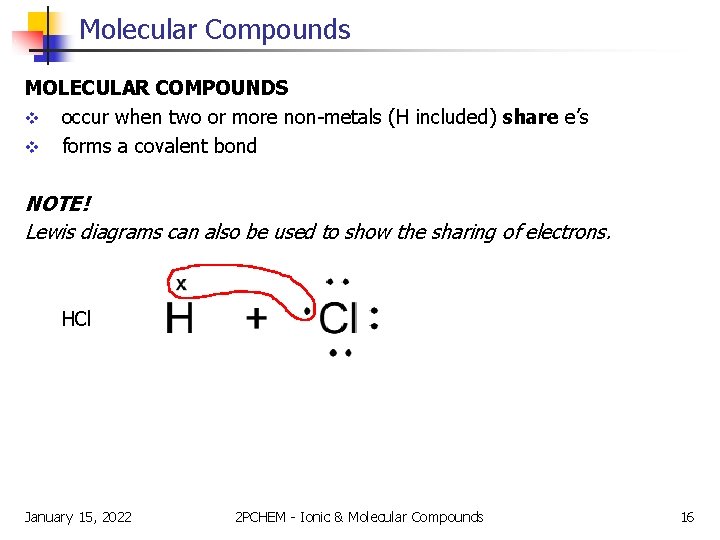
Molecular Compounds MOLECULAR COMPOUNDS v occur when two or more non-metals (H included) share e’s v forms a covalent bond NOTE! Lewis diagrams can also be used to show the sharing of electrons. HCl January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 16

Molecular Compounds Molecular compounds also have a number of properties in common. PROPERTIES OF MOLECULAR COMPOUNDS v can be solids, liquids, or gases at room temperature v low melting points (i. e. weak force of attraction between neutral molecules) v form solutions that do not conduct electricity NOTE! While the force of attraction between the molecules is weak (since the molecules are neutral), it is important to remember that the bonds between the atoms in the molecule itself are strong. January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 17

Molecular Compounds PRACTICE 5. the following pairs of atoms: (a) H + H (H 2) (b) N + N (N 2) January 15, 2022 (c) C + O (d) H + O 2 PCHEM - Ionic & Molecular Compounds (CO 2) (H 2 O) 18

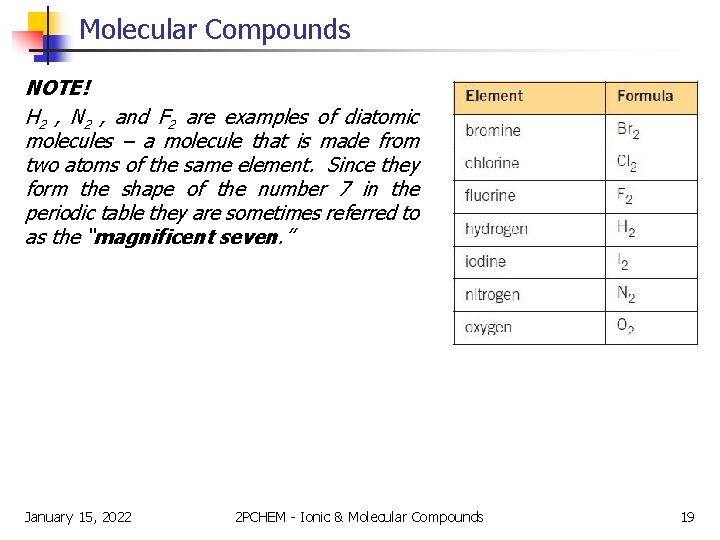
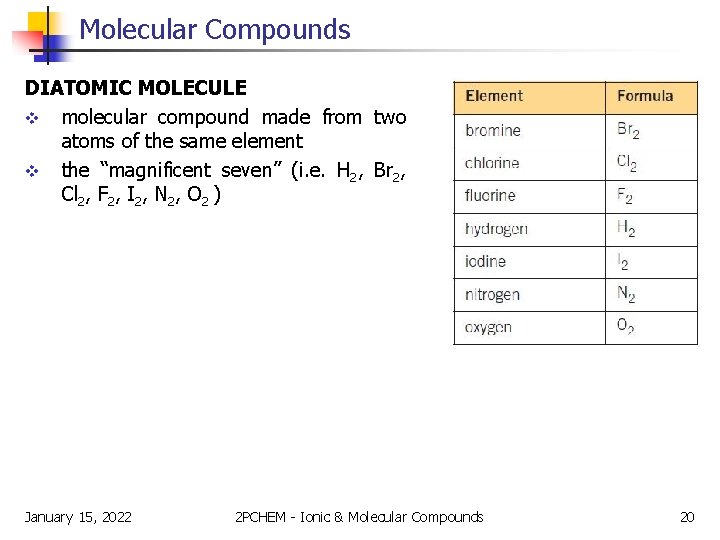
Molecular Compounds NOTE! H 2 , N 2 , and F 2 are examples of diatomic molecules – a molecule that is made from two atoms of the same element. Since they form the shape of the number 7 in the periodic table they are sometimes referred to as the “magnificent seven. ” January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 19

Molecular Compounds DIATOMIC MOLECULE v molecular compound made from two atoms of the same element v the “magnificent seven” (i. e. H 2, Br 2, Cl 2, F 2, I 2, N 2, O 2 ) January 15, 2022 2 PCHEM - Ionic & Molecular Compounds 20
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Types of reactions
Types of reactions Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Hpps logo
Hpps logo Sistema nervioso central
Sistema nervioso central Snc1d chemistry
Snc1d chemistry Snc lajaa venissieux
Snc lajaa venissieux Snc società
Snc società Síndrome de kleeblattschädel
Síndrome de kleeblattschädel Paul mcenaney
Paul mcenaney What are redox reactions examples
What are redox reactions examples Ccea gce chemistry practical support
Ccea gce chemistry practical support Vcaa chemistry study design
Vcaa chemistry study design Ccea gce chemistry practical support
Ccea gce chemistry practical support Types of reactions chemistry
Types of reactions chemistry 5 types of reactions in chemistry
5 types of reactions in chemistry