SNC 2 P CHEMISTRY CHEMICAL REACTIONS THEIR PRACTICAL

















- Slides: 20

SNC 2 P CHEMISTRY CHEMICAL REACTIONS & THEIR PRACTICAL APPLICATIONS � Chemical Tests – Practice Formal Lab (P. ~)

Activity: Practice Formal Lab (QHMMORCA) Recall that one of the most important characteristics of scientific investigation is that scientists share their information. Clear and accurate communication is essential for sharing information. It is important to share not only the evidence, but also the process by which evidence was obtained. If the investigation is to be repeated by others, it is just as important to share the design and procedures. NOTE! In this course, we will use the QHMMORCA method (refer to the “Lab Report Outline” handout). December 26, 2021 2 PCHEM - Chemical Tests 1

Activity: Practice Formal Lab (QHMMORCA) INSTRUCTIONS A. As a class, you will write up a “practice” formal report of the experiment you just completed. B. When you are finished be sure to submit your “practice” formal report to your teacher so that they can check it for completeness and accuracy. NOTE! You may use either a pen or pencil for this practice report but a real/true formal report will require the use of a pen or computer. The use of a ruler when designing your observation tables will also be necessary. December 26, 2021 2 PCHEM - Chemical Tests 2

Activity: Practice Formal Lab (QHMMORCA) LET’S BEGIN!! December 26, 2021 2 PCHEM - Chemical Tests 3

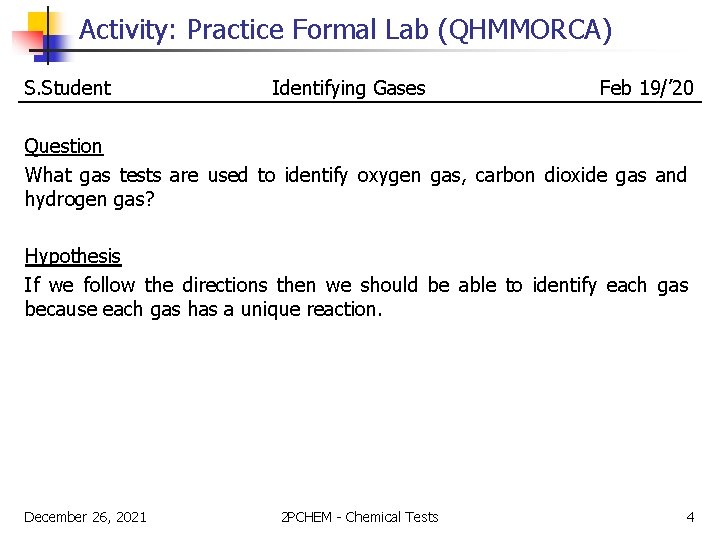
Activity: Practice Formal Lab (QHMMORCA) S. Student Identifying Gases Feb 19/’ 20 Question What gas tests are used to identify oxygen gas, carbon dioxide gas and hydrogen gas? Hypothesis If we follow the directions then we should be able to identify each gas because each gas has a unique reaction. December 26, 2021 2 PCHEM - Chemical Tests 4

Activity: Practice Formal Lab (QHMMORCA) Materials NOTE! If the list of materials are given to you (i. e. in a handout or from the textbook) then you can just use the phrase, Refer to the materials as given to us in the handout/textbook. If necessary, be sure to make note of any changes made to the materials. December 26, 2021 2 PCHEM - Chemical Tests 5

Activity: Practice Formal Lab (QHMMORCA) Materials In this case we can’t do that so, . . . December 26, 2021 2 PCHEM - Chemical Tests 6

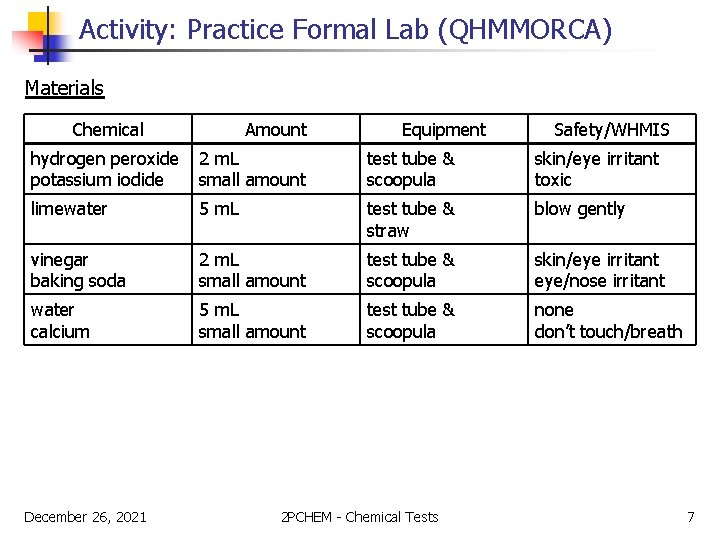
Activity: Practice Formal Lab (QHMMORCA) Materials Chemical Amount Equipment Safety/WHMIS hydrogen peroxide potassium iodide 2 m. L small amount test tube & scoopula skin/eye irritant toxic limewater 5 m. L test tube & straw blow gently vinegar baking soda 2 m. L small amount test tube & scoopula skin/eye irritant eye/nose irritant water calcium 5 m. L small amount test tube & scoopula none don’t touch/breath December 26, 2021 2 PCHEM - Chemical Tests 7

Activity: Practice Formal Lab (QHMMORCA) Method NOTE! Similar to Materials, if the method is given to you (i. e. in a handout or from the textbook) then you can just use the phrase, Refer to the method as given to us in the handout/textbook. If necessary, be sure to make note of any changes made to the method. December 26, 2021 2 PCHEM - Chemical Tests 8

Activity: Practice Formal Lab (QHMMORCA) Method In this case we can do this so, . . . December 26, 2021 2 PCHEM - Chemical Tests 9

Activity: Practice Formal Lab (QHMMORCA) Method Refer to the method (steps 1 -22) as given to us in the “Identifying Gases” handout. December 26, 2021 2 PCHEM - Chemical Tests 10

Activity: Practice Formal Lab (QHMMORCA) Observations NOTE! If your observation table is too large to fit in the remaining space then you can direct the reader to the location of the table by using the phrase, Refer to page __. December 26, 2021 2 PCHEM - Chemical Tests 11

Activity: Practice Formal Lab (QHMMORCA) Observations In this case we don’t need to do this so, . . . December 26, 2021 2 PCHEM - Chemical Tests 12

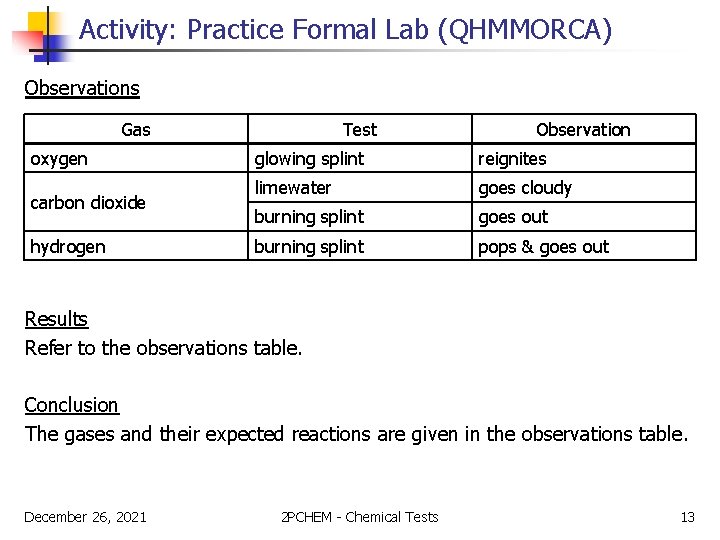
Activity: Practice Formal Lab (QHMMORCA) Observations Gas oxygen carbon dioxide hydrogen Test Observation glowing splint reignites limewater goes cloudy burning splint goes out burning splint pops & goes out Results Refer to the observations table. Conclusion The gases and their expected reactions are given in the observations table. December 26, 2021 2 PCHEM - Chemical Tests 13

Activity: Practice Formal Lab (QHMMORCA) Analysis NOTE! This is the section where you would answer any additional questions asked. In this case, the next slide contains a series of questions that we will answer together. It is not necessary to copy the questions down, but when you are answering them you should always use a lead-in statement. For example, . . . December 26, 2021 2 PCHEM - Chemical Tests 14

Activity: Practice Formal Lab (QHMMORCA) Analysis 1. How would you test for the gas produced in each of the following, and what observations would you expect to make? (a) A can of pop fizzes. (b) A nail added to a strong acid produces a combustible gas. 2. Hydrogen’s low density makes it useful for weather balloons. Why is hydrogen not used in blimps that carry people? December 26, 2021 2 PCHEM - Chemical Tests 15

Activity: Practice Formal Lab (QHMMORCA) Analysis 1. (a) I would test the gas released by the can of pop for carbon dioxide using either (i) limewater which would turn cloudy or (ii) a burning splint which would go out. (b) I would test the gas released by the nail and acid for hydrogen gas using a burning splint which would “pop” and put the flame out. 2. Hydrogen gas is not used in blimps that carry people because the gas is highly flammable and explosive. NOTE! When you are finished submit the following stapled together: Page 1 Lab Report Outline (QHMMORCA) Handout Page 2 Identifying Gases Handout Page 3 & … Your Practice Formal Lab December 26, 2021 2 PCHEM - Chemical Tests 16

Activity: Practice Formal Lab (QHMMORCA) NOTE! This finishes this Power. Point presentation. The next two slides are for those classes where you wish to have the students practice writing the method in third person, past tense. December 26, 2021 2 PCHEM - Chemical Tests 17

Activity: Practice Formal Lab (QHMMORCA) Method Oxygen Gas 1. About 2 m. L of hydrogen peroxide was added to a very small amount of potassium iodide powder in a test tube. 2. A glowing splint was then inserted into the mouth of the test tube. 3. Observations were made and recorded. 4. The contents were discarded and the test tube cleaned. Carbon Dioxide (Part 1) 5. About 5 m. L of limewater was placed in a test tube. 6. A straw was used to blow into the limewater. 7. Observations were made and recorded. 8. The contents were discarded and the test tube cleaned. December 26, 2021 2 PCHEM - Chemical Tests 18

Activity: Practice Formal Lab (QHMMORCA) Carbon Dioxide (Part 2) 9. About 2 m. L of vinegar was added to a very small amount of baking soda in a test tube. 10. A burning splint was then inserted into the mouth of the test tube. 11. Observations were made and recorded. 12. The contents were discarded and the test tube cleaned. Hydrogen Gas 13. About 5 m. L of water was added to a small amount of calcium in a test tube. A large test tube was then placed upside down over this test tube to trap any gas produced. 14. After 1 minute the large test tube was removed and then a burning splint was then inserted into the mouth of this test tube. 15. Observations were made and recorded. 16. The contents were discarded and the test tubes cleaned. December 26, 2021 2 PCHEM - Chemical Tests 19
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Snc set symbol
Snc set symbol Rombencefalo
Rombencefalo Water density
Water density Snc lajaa venissieux
Snc lajaa venissieux Snc
Snc Síndrome de pfeiffer tipo 2
Síndrome de pfeiffer tipo 2 Snc database
Snc database Example of oxidation reduction reaction
Example of oxidation reduction reaction Salters advanced chemistry
Salters advanced chemistry Vce chemistry research investigation
Vce chemistry research investigation Ccea gce chemistry practical support
Ccea gce chemistry practical support Types of reactions chemistry
Types of reactions chemistry