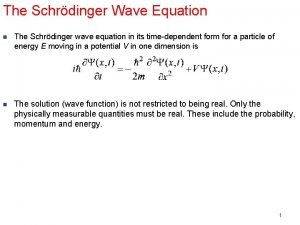
SIGNIFICANCE OF QUANTUM MECHANICS MOST IMPORTANT SIGNIFICANCE Quantum






- Slides: 24

SIGNIFICANCE OF QUANTUM MECHANICS

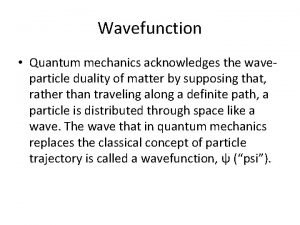
MOST IMPORTANT SIGNIFICANCE • Quantum Mechanics was able to describe the wave particle duality. • 1. Blackbody Radiation • 2. Photoelectric Effect • 3. Compton Effect • 4. de Broglie Equation • It also revolutionized the way we understand Chemistry.

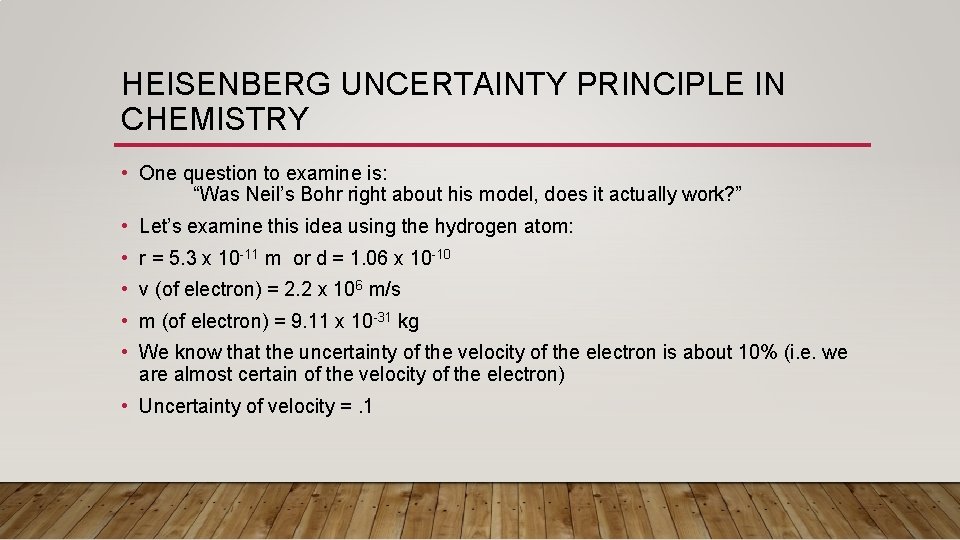
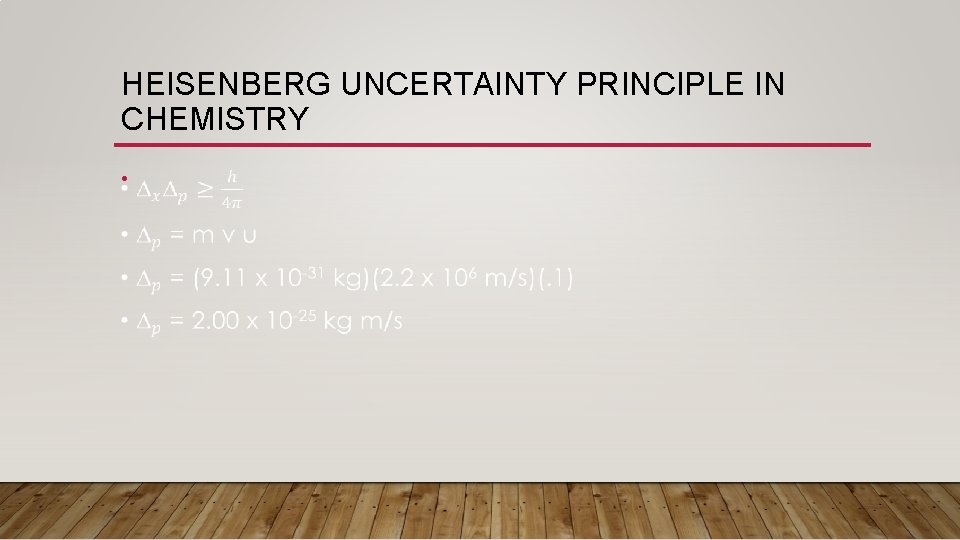
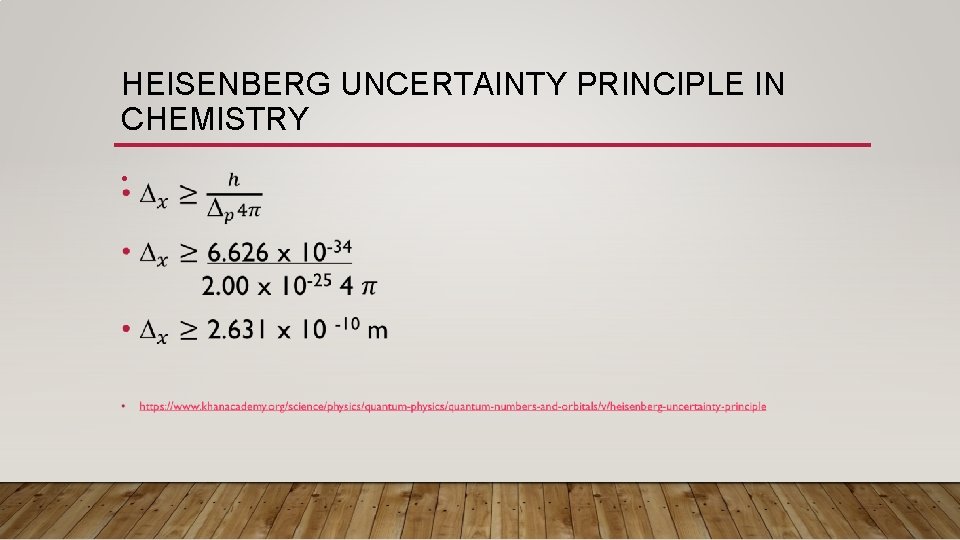
HEISENBERG UNCERTAINTY PRINCIPLE IN CHEMISTRY • One question to examine is: “Was Neil’s Bohr right about his model, does it actually work? ” • Let’s examine this idea using the hydrogen atom: • r = 5. 3 x 10 -11 m or d = 1. 06 x 10 -10 • v (of electron) = 2. 2 x 106 m/s • m (of electron) = 9. 11 x 10 -31 kg • We know that the uncertainty of the velocity of the electron is about 10% (i. e. we are almost certain of the velocity of the electron) • Uncertainty of velocity =. 1

HEISENBERG UNCERTAINTY PRINCIPLE IN CHEMISTRY •

HEISENBERG UNCERTAINTY PRINCIPLE IN CHEMISTRY •

HEISENBERG UNCERTAINTY PRINCIPLE IN CHEMISTRY • WOWZA, Bohr’s model has some errors. The distance that an electron travels around the nucleus is larger than 1. 6 x 10 -10 m, it is actually 2. 631 x 10 -10 m. • So what does this mean? • Well thanks to Heisenberg we know that the electron is located somewhere around the atom and has a diameter of 2. 631 x 10 -10 m.

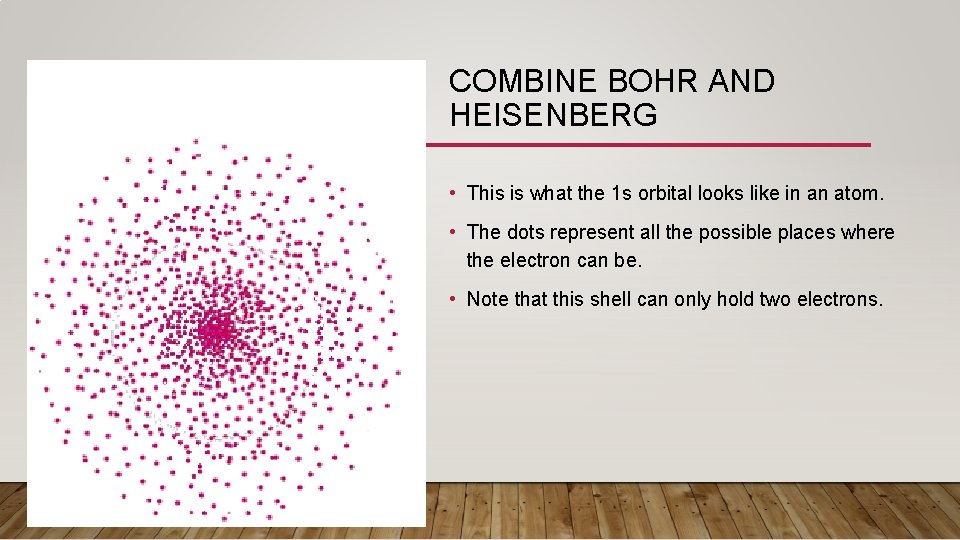
COMBINE BOHR AND HEISENBERG • This is what the 1 s orbital looks like in an atom. • The dots represent all the possible places where the electron can be. • Note that this shell can only hold two electrons.

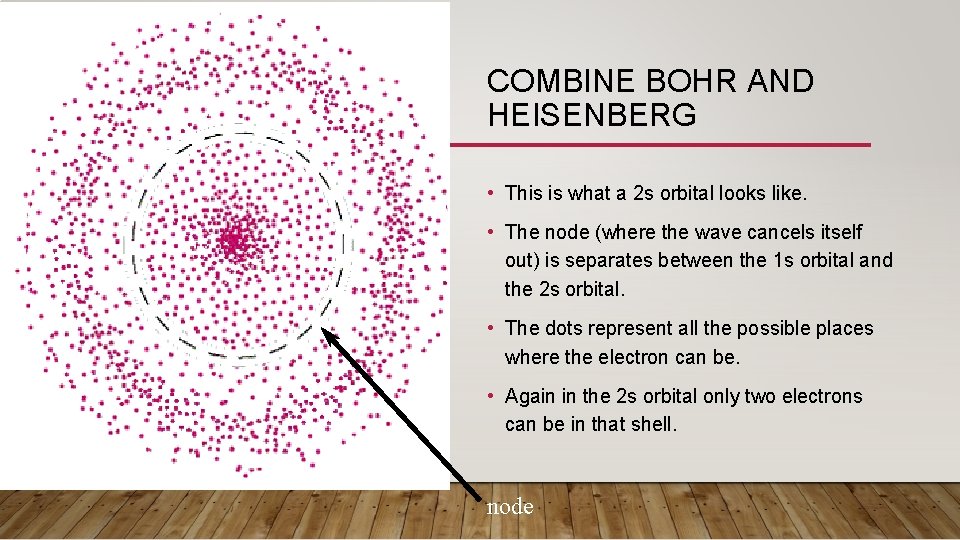
COMBINE BOHR AND HEISENBERG • This is what a 2 s orbital looks like. • The node (where the wave cancels itself out) is separates between the 1 s orbital and the 2 s orbital. • The dots represent all the possible places where the electron can be. • Again in the 2 s orbital only two electrons can be in that shell. node

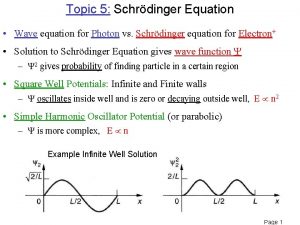
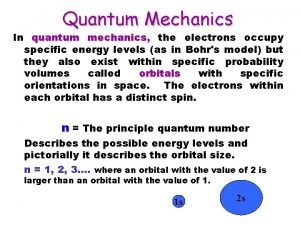
QUANTUM MECHANICS SOLVES WHAT AN ATOM LOOKS LIKE • Through a series of complex calculations, physicists and chemists where able to create four specific numbers of importance that help us understand how the electrons move in an atom. • 1. Principle Quantum Number (n) – size and energy level of the orbit. • 1, 2, 3, 4, 5, 6, 7 • Each number identifies the row on the periodic table.

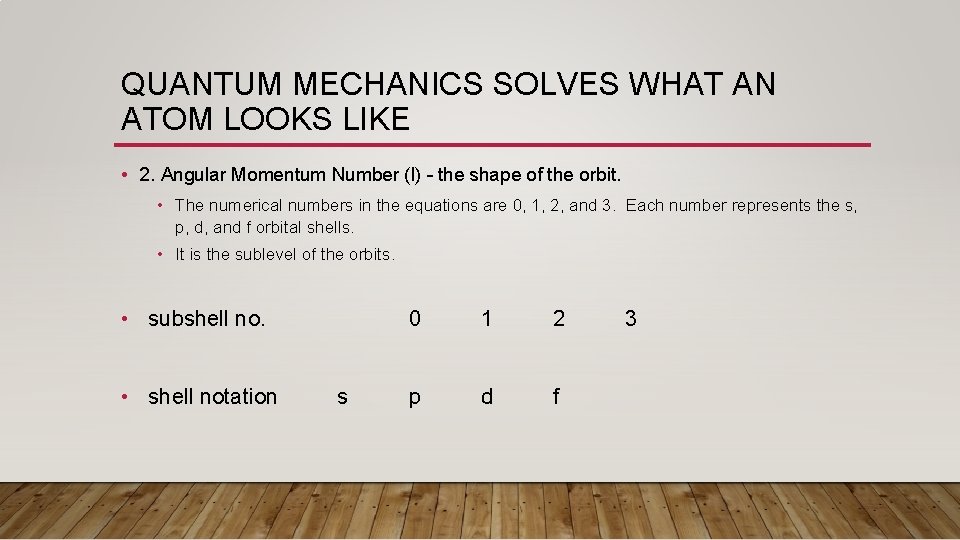
QUANTUM MECHANICS SOLVES WHAT AN ATOM LOOKS LIKE • 2. Angular Momentum Number (l) – the shape of the orbit. • The numerical numbers in the equations are 0, 1, 2, and 3. Each number represents the s, p, d, and f orbital shells. • It is the sublevel of the orbits. • subshell no. • shell notation s 0 1 2 p d f 3

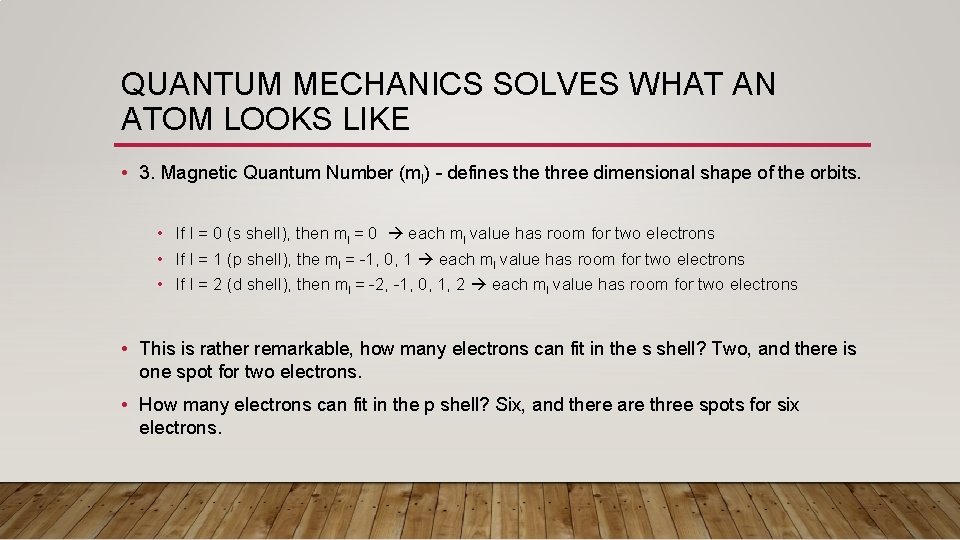
QUANTUM MECHANICS SOLVES WHAT AN ATOM LOOKS LIKE • 3. Magnetic Quantum Number (ml) – defines the three dimensional shape of the orbits. • If l = 0 (s shell), then ml = 0 each ml value has room for two electrons • If l = 1 (p shell), the ml = -1, 0, 1 each ml value has room for two electrons • If l = 2 (d shell), then ml = -2, -1, 0, 1, 2 each ml value has room for two electrons • This is rather remarkable, how many electrons can fit in the s shell? Two, and there is one spot for two electrons. • How many electrons can fit in the p shell? Six, and there are three spots for six electrons.

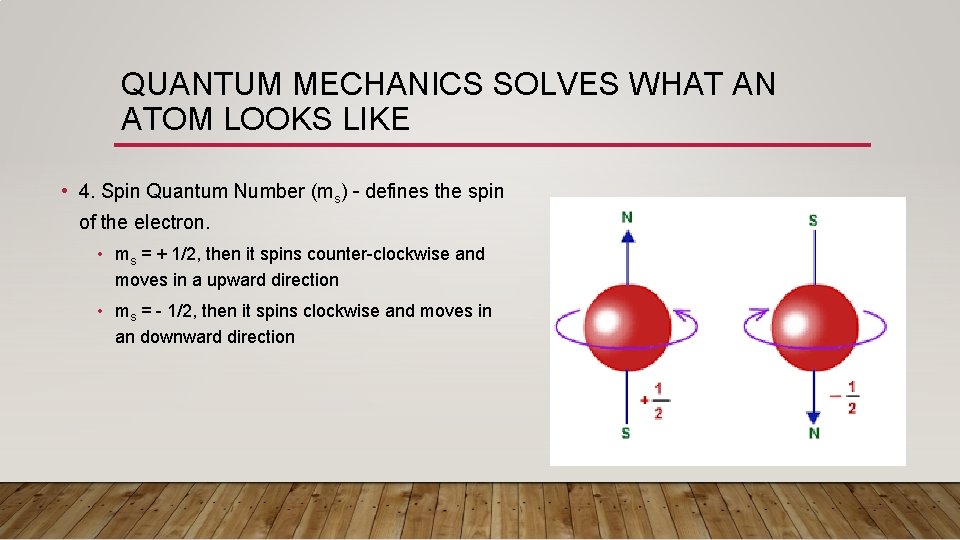
QUANTUM MECHANICS SOLVES WHAT AN ATOM LOOKS LIKE • 4. Spin Quantum Number (ms) – defines the spin of the electron. • ms = + 1/2, then it spins counter-clockwise and moves in a upward direction • ms = - 1/2, then it spins clockwise and moves in an downward direction

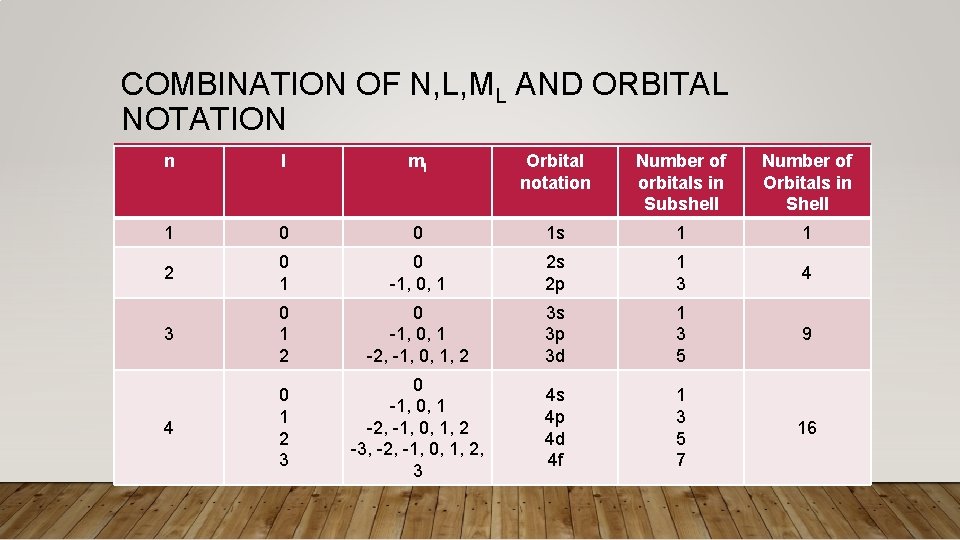
COMBINATION OF N, L, ML AND ORBITAL NOTATION n l ml Orbital notation Number of orbitals in Subshell Number of Orbitals in Shell 1 0 0 1 s 1 1 2 0 1 0 -1, 0, 1 2 s 2 p 1 3 4 3 0 1 2 0 -1, 0, 1 -2, -1, 0, 1, 2 3 s 3 p 3 d 1 3 5 9 4 0 1 2 3 0 -1, 0, 1 -2, -1, 0, 1, 2 -3, -2, -1, 0, 1, 2, 3 4 s 4 p 4 d 4 f 1 3 5 7 16

HOW TO FILL THE SHELLS • Note that 3 d starts with Scantium, 4 d starts with Yitrium, 5 d starts with Lanthanium, and 6 d starts with Actinium. • Note that 4 f starts with Cerium and 5 f starts with Thorium.

FINDING THE ELEMENT USING QUANTUM NUMBERS • First find the row number you are in - 1, 2, 3, 4…. • Secondly find the subshell you are in - 0(s), 1(p), 2(d), 3(f) • Thirdly find the subshell location you are in by drawing out how many subshells there are in the shell orbit you are in. Example for subshell p draw three lines and number them -1, 0, 1. For subshell d number them -2, -1, 0, 1, 2. • Finally, fill the electron subshells with electron until you get to the proper +1/2 or 1/2 spin.

FEW EXAMPLES • If n = 2, l = 0, ml = 0 and ms = -1/2 what element is it? • Berylium • If n = 3, l = 2, ml = -1 and ms = -1/2 what element is it? • Cobalt • If n = 2, l = 1, ml = 1 and ms = -1/2 what element is it? • Neon

MORE EXAMPLES • If n = 4, l = 2, ml = 2 and ms = 1/2 what element is it? • Technellium • If n = 4, l = 1, ml = -1 and ms = 1/2 what element is it? • Gallium

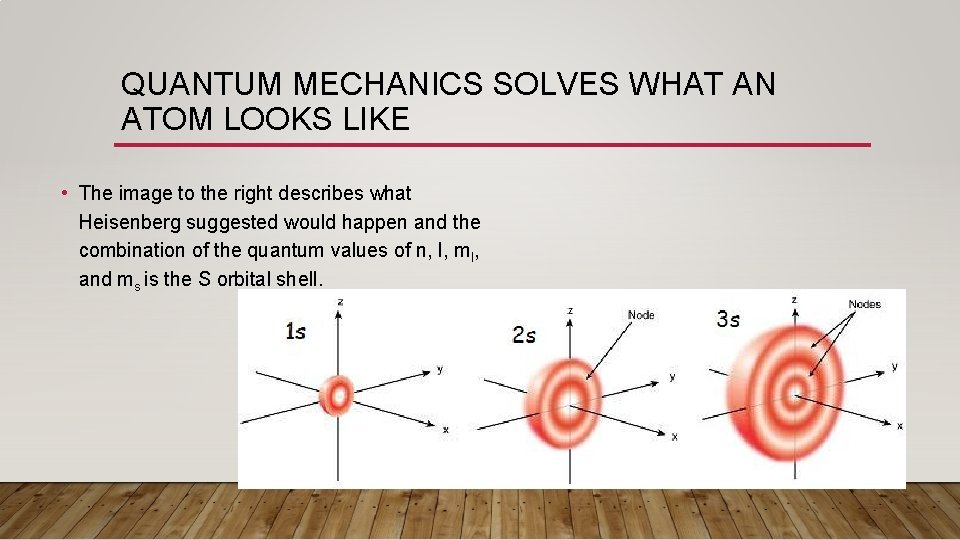
QUANTUM MECHANICS SOLVES WHAT AN ATOM LOOKS LIKE • The image to the right describes what Heisenberg suggested would happen and the combination of the quantum values of n, l, ml, and ms is the S orbital shell.

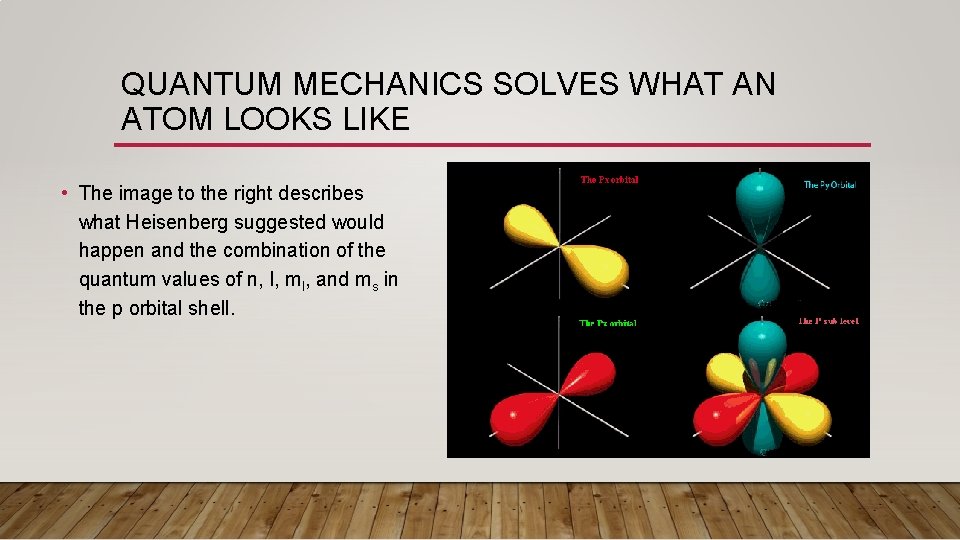
QUANTUM MECHANICS SOLVES WHAT AN ATOM LOOKS LIKE • The image to the right describes what Heisenberg suggested would happen and the combination of the quantum values of n, l, ml, and ms in the p orbital shell.

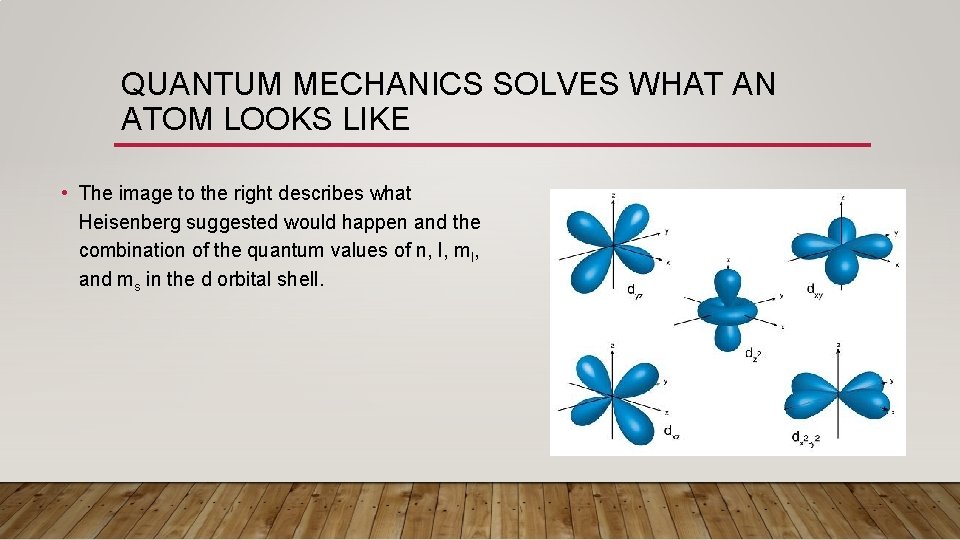
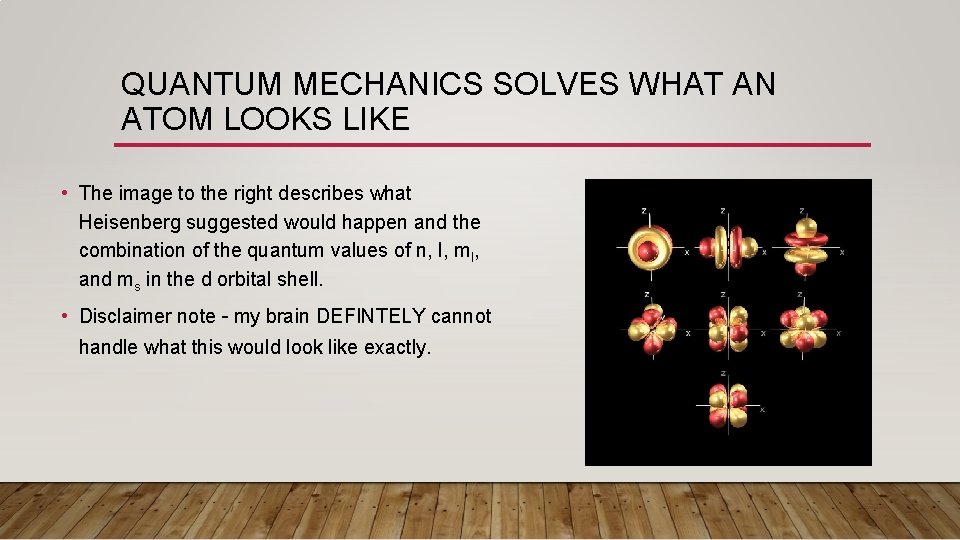
QUANTUM MECHANICS SOLVES WHAT AN ATOM LOOKS LIKE • The image to the right describes what Heisenberg suggested would happen and the combination of the quantum values of n, l, ml, and ms in the d orbital shell.

QUANTUM MECHANICS SOLVES WHAT AN ATOM LOOKS LIKE • The image to the right describes what Heisenberg suggested would happen and the combination of the quantum values of n, l, ml, and ms in the d orbital shell. • Disclaimer note – my brain DEFINTELY cannot handle what this would look like exactly.

SIGNIFICANCE OF QUANTUM MECHANICS • All the things we know to understand in life – electronics, chemistry, biology, organic molecules, the table you are writing on and etc… has all been calculated through mathematical equations. • If you think about it, everything we see, observe and do has been calculated mathematically. • There is a great movie called “What the Bleep” where they use our understanding of quantum mechanics to actually understand depression and what actually happens in our brains.

SCIENCE AND THE CORE COMPETENCIES • Using mathematics we deduce something is happening – critical thinking. • Then we use the math to draw out and describe what we are observing - creative thinking. • We then take make a personal decision to either share this information or not – personal awareness. • We then decide what kind of responsibility we have in sharing this information with society – social responsibility (solar panels, atomic bomb, computers, electronics, biological warfare, etc…) • In sharing this information we have to write it out so our peers can agree with our findings, and then express the ideas so the regular lay person can understand - communications. • In doing all this we then reflect on what we have done – personal and cultural identity.

CONGRATS • You have come to the end of the Physics 11 journey. • Now lets get in a circle and discuss the year that has unfolded.
 Classical mechanics
Classical mechanics Quantum physics vs quantum mechanics
Quantum physics vs quantum mechanics Example of a news story
Example of a news story Inverted pyramid in news writing
Inverted pyramid in news writing Least important to most important
Least important to most important Expectation value of hermitian operator
Expectation value of hermitian operator Expectation value of energy in quantum mechanics
Expectation value of energy in quantum mechanics Incident wave equation
Incident wave equation Expectation value in quantum mechanics
Expectation value in quantum mechanics Schrodingers cay
Schrodingers cay Quantum mechanics in your face
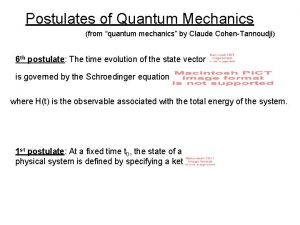
Quantum mechanics in your face Postulates of quantum mechanics
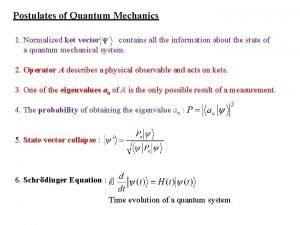
Postulates of quantum mechanics Postulates of quantum mechanics
Postulates of quantum mechanics Operators in quantum mechanics
Operators in quantum mechanics Operators in quantum mechanics
Operators in quantum mechanics Susan cartwright sheffield
Susan cartwright sheffield Operators in quantum mechanics
Operators in quantum mechanics Schroendiger
Schroendiger Ap physics quantum mechanics
Ap physics quantum mechanics Instantons
Instantons Expectation value in quantum mechanics
Expectation value in quantum mechanics Mathematical tools of quantum mechanics
Mathematical tools of quantum mechanics Quantum mechanics in three dimensions
Quantum mechanics in three dimensions Quantum mechanics basics
Quantum mechanics basics Grandfather paradox
Grandfather paradox