Section 1 Fundamentals of Nutrition Copyright 2003 Delmar

























- Slides: 37

Section 1 Fundamentals of Nutrition Copyright © 2003 Delmar Learning, a Thomson Learning company

Chapter 9 Water Copyright © 2003 Delmar Learning, a Thomson Learning company

Objectives § § § Chapter 9 Describe the functions of water in the body Explain fluid balance and its maintenance Name causes and consequences of water depletion Give causes and consequences of positive fluid balance Describe the acid-base balance of the human body Copyright © 2003 Delmar Learning, a Thomson Learning company 3

Facts Humans can live about 8 weeks without food. One can live only a few days without water. Water is in all body cells. 50 -60% body weight of normal adults. Percentage is highest in newborns; decreases with age. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 4

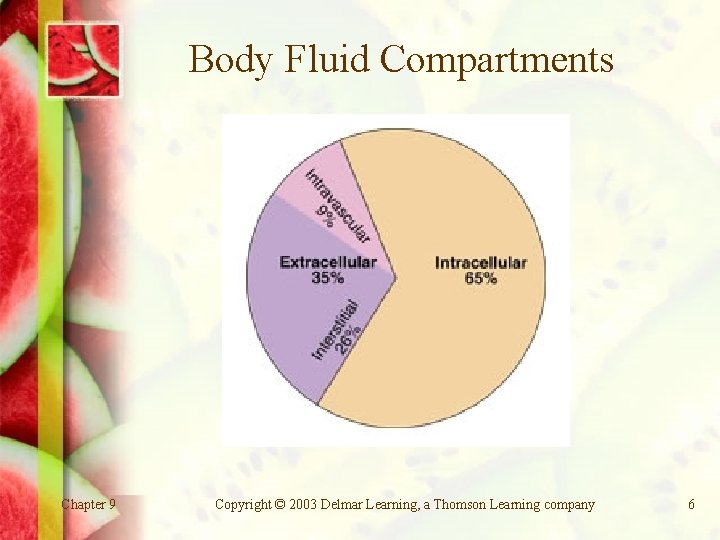
Facts Two basic compartments • • Intracellular fluid (ICF): within cells; 65% of total body fluid. Extracellular fluid (ECF): outside cells; 35% of total body fluid. - Chapter 9 Divided into intravascular fluid (in blood stream) and interstitial fluid (between cells) Copyright © 2003 Delmar Learning, a Thomson Learning company 5

Body Fluid Compartments Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 6

Functions Major component of blood plasma. Solvent for nutrients and waste products. Necessary for hydrolysis of nutrients. Essential for metabolism. Lubricant in joints and digestion. Cools the body through perspiration. Provides some mineral elements. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 7

Sources Drinking water is the best source. Beverages are second-best source. Other sources include fruits, vegetables, soups, milk, and gelatin desserts. Energy metabolism produces water. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 8

Estimated Daily Fluid Intake for an Adult Ingested liquids Water in foods Water from oxidation Total Chapter 9 1, 500 ml 700 ml 2, 400 ml Copyright © 2003 Delmar Learning, a Thomson Learning company 9

Fluid and Electrolyte Balance Electrolytes are measured in milliequivalents (m. Eq/L). Sensible (noticeable) water loss is water lost through urine. Insensible (unnoticeable) water loss is in feces, perspiration, and respiration. Waste products of metabolism excreted in the form of urine (500 ml of water each day). Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 10

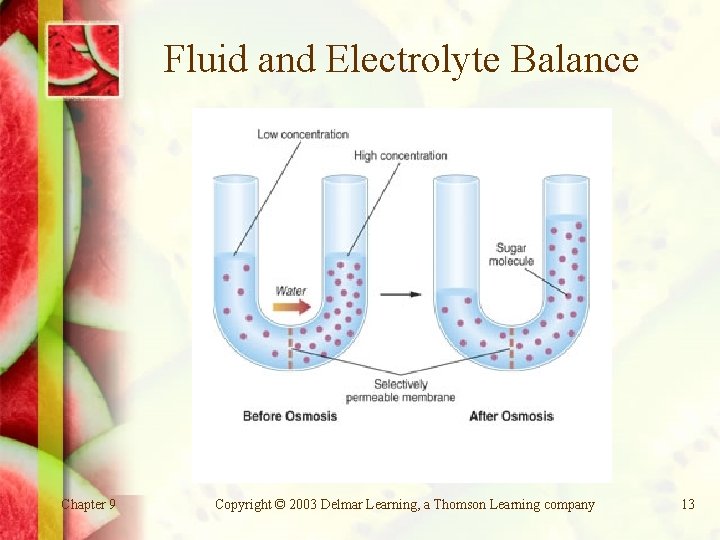
Fluid and Electrolyte Balance Solute: substance dissolved in a solution. Osmosis: water flows from the side with the lesser amount of solute to the side with the greater solute concentration. Sodium, chloride, and potassium maintain the balance between intracellular and extracellular fluids. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 11

Fluid and Electrolyte Balance Potassium is the principal electrolyte in intracellular fluid. Sodium is the principal electrolyte in extracellular fluid. Osmolality measures particles in a solution. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 12

Fluid and Electrolyte Balance Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 13

Fluid and Electrolyte Balance When electrolytes in extracellular fluid are increased, ICF moves to the ECF to equalize the concentration of electrolytes on both sides of the membrane. Reduces the amount of water in the cells. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 14

Fluid and Electrolyte Balance Hypothalamus stimulates pituitary gland to excrete ADH (antidiuretic hormone). ADH causes kidneys to reabsorb water. Thirst causes healthy person to drink fluids. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 15

Fluid and Electrolyte Balance When sodium in ECF is reduced, water flows from ECF into cells, causing cellular edema. Adrenal glands secrete aldosterone, which triggers kidneys to increase the amount of sodium reabsorbed. When the missing sodium is replaced in the ECF, excess water moves back to the ECF and edema is relieved. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 16

Fluid and Electrolyte Balance Amount of water use varies, depending on age, size, activity, environmental temperature, and physical condition. Average adult requirement is 1 ml for every kcal in food consumed. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 17

Stop and Share How many glasses of fluid would be required for an adult eating 1, 800 kcal/day? Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 18

Stop and Share 1 ml × 1, 800 kcal = 1, 800 cc 240 oz = 7. 5 glasses of water It is recommended that adults drink eight 8 -ounce glasses of fluid a day. Youth, fever, diarrhea, unusual perspiration, and hyperthyroidism increase the requirement. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 19

Dehydration Amount of water in the body is inadequate. Caused by inadequate intake or abnormal loss. Loss can occur from severe diarrhea, vomiting, hemorrhage, burns, diabetes mellitus, excessive perspiration, excessive urination, or the use of certain medications such as diuretics. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 20

Dehydration Symptoms of dehydration include low blood pressure, thirst, dry skin, fever, and mental disorientation. As water is lost, electrolytes are also lost. Treatment involves replacement of electrolytes and fluids. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 21

Dehydration 10% loss can cause serious problems. Blood volume and nutrient absorption are reduced, and kidney function is upset. 20% loss can cause circulatory failure and death. Infants are at high risk for dehydration when fever, vomiting, and diarrhea occur. Treatment involves IV fluids. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 22

Dehydration Thirst sensation lags behind the body’s need for water, especially in the elderly, children, athletes, and the ill. Feeling thirsty is not a reliable indicator of when the body needs water. Fluids should be drunk throughout the day to prevent dehydration. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 23

Dehydration Failure to replace water lost through perspiration could lead to one of the four stages of heat illness: • • Chapter 9 Heat fatigue Heat cramp Heat exhaustion Heat stroke Copyright © 2003 Delmar Learning, a Thomson Learning company 24

Signs of Dehydration Health history reveals inadequate intake of fluids Decrease in urine output Weight loss Eyes appear sunken Tongue has increased furrows and fissures Oral mucous membranes are dry Decreased skin turgor Changes in neurological status Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 25

Excess Water Accumulation Positive water balance–more water taken in than excreted; edema results. Hypothyroidism, congestive heart failure, hypoproteinemia, some infections, some cancers, and some renal conditions can cause water retention because sodium is not being excreted normally. Fluids and sodium may then be restricted. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 26

Acid-base Balance Regulation of hydrogen ions Acid gives off hydrogen ions Base picks up hydrogen ions Acidic substances–p. H 1 to 7 Alkaline substances–p. H 7 to 14 p. H 7 is considered neutral Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 27

Acid-base Balance Blood plasma–p. H 7. 35 to 7. 45 Intracellular fluid–p. H 6. 8 Kidneys maintain acid-base balance What a person eats affects the acidity not of the body but of the urine. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 28

Buffer Systems Regulate hydrogen ion content in body fluids Mixture of a weak acid and a strong base Normal buffer system–ratio of base to acid 20: 1 Carbonic acid and sodium bicarbonate forms the body’s main buffer system. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 29

Buffer Systems Carbonic acid moves easily to buffer a strong alkali, and sodium bicarbonate moves easily to buffer a strong acid. Amounts are easily adjusted by the lungs and kidneys to suit needs. End products of metabolism are carbon dioxide and water, and together they can form carbonic acid. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 30

Buffer Systems The medulla oblongata in the brain causes the breathing rate to increase if the amount of carbon dioxide is more concentrated than it should be. This increases the rate at which the body rids itself of carbon dioxide. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 31

Buffer Systems Excess sodium bicarbonate is excreted via the kidneys. The kidneys can excrete urine from p. H 4. 5 to p. H 8. The p. H of average urine is 6. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 32

Acidosis and Alkalosis Renal failure, uncontrolled diabetes mellitus, starvation, or severe diarrhea can cause acidosis. Alkalosis can occur when the body has suffered a loss of hydrochloric acid from severe vomiting or has ingested too much alkali, such as too many antacid tablets. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 33

Stop and Share A client is unhappy with her low sodium, fluid restricted diet. How can the health care professional best help the client? Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 34

Stop and Share Discuss realistic ways of planning menus for her and with her. Base menus on good nutrition, the client’s normal habits and desires. Review former diet with the client. Point out high-salt and high-liquid foods and present alternative foods in a positive manner. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 35

Conclusion Water is a component of all tissues. Solvent for nutrients and body wastes. Provides transport for both. Essential for hydrolysis, lubrication, and maintenance of normal temperature. Best sources are water, beverages, fruits, vegetables, soups, and water-based desserts. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 36

Conclusion Dehydration may result from lack of water. Positive water balance is an excess accumulation of water in the body. Acid-base balance is the regulation of hydrogen ions in the body. Healthy people have intricate maintenance systems for fluid, electrolytes, and acidbase balance. Chapter 9 Copyright © 2003 Delmar Learning, a Thomson Learning company 37
 Copyright 2003
Copyright 2003 2008 pearson education inc
2008 pearson education inc Copyright 2003
Copyright 2003 Copyright 2003
Copyright 2003 Fundamentals of nursing nutrition
Fundamentals of nursing nutrition Nutrition fundamentals of nursing
Nutrition fundamentals of nursing Chapter 6:2 interpreting word parts
Chapter 6:2 interpreting word parts 2009 delmar cengage learning
2009 delmar cengage learning Clinical conditions chapter 1 medical terminology
Clinical conditions chapter 1 medical terminology 2009 delmar cengage learning
2009 delmar cengage learning Delmar isotonic
Delmar isotonic Delmar tsi
Delmar tsi Delmar international (thailand) co. ltd
Delmar international (thailand) co. ltd Thomson delmar learning
Thomson delmar learning Chapter 6 skeletal system
Chapter 6 skeletal system Chapter 13 medical math
Chapter 13 medical math 2009 delmar cengage learning
2009 delmar cengage learning Delmar larsen
Delmar larsen Delmar cengage learning instructor resources
Delmar cengage learning instructor resources 38-2 the process of digestion
38-2 the process of digestion Section 38-1 food and nutrition
Section 38-1 food and nutrition Chapter 38 digestive and excretory systems answer key
Chapter 38 digestive and excretory systems answer key Microsoft word 2003 tutorial
Microsoft word 2003 tutorial Montreux record meaning
Montreux record meaning Sbs 2003 cals
Sbs 2003 cals Windows 2003 service pack 1
Windows 2003 service pack 1 Where did wilson rawls live
Where did wilson rawls live 2003 ub
2003 ub Visio 2003 viewer
Visio 2003 viewer Upgrade 2003 to 2008
Upgrade 2003 to 2008 R v ruffell 2003
R v ruffell 2003 Gianfranco gentile
Gianfranco gentile Tesla founded 2003
Tesla founded 2003 Scale development steps
Scale development steps Spring, summer, fall, winter... and spring cast
Spring, summer, fall, winter... and spring cast 2003 ub
2003 ub S78 soa 2003
S78 soa 2003 Legge moratti 53/2003 sintesi
Legge moratti 53/2003 sintesi