Rates of Reaction Reactions can occur at different

























- Slides: 36

Rates of Reaction

• Reactions can occur at different speeds • Fast reaction – lighted taper held up to balloon • Slow reaction – apple ripening • Rate of reaction is the change in concentration per unit time of any one reactant or product.

Factors affecting rates of reaction 1) nature of reactants 2) particle size 3) concentration 4) temperature 5) catalysts

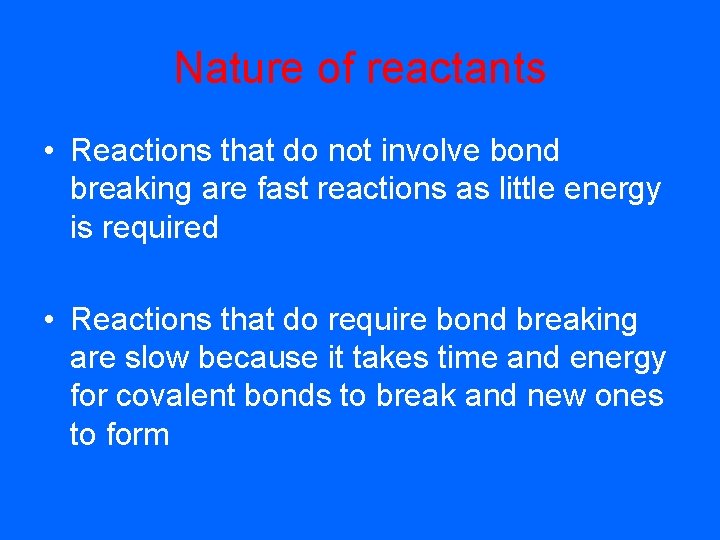
Nature of reactants • Reactions that do not involve bond breaking are fast reactions as little energy is required • Reactions that do require bond breaking are slow because it takes time and energy for covalent bonds to break and new ones to form

Particle size • The smaller the particle size the more surface area there is available • The more surface area there is available means the reaction can occur faster

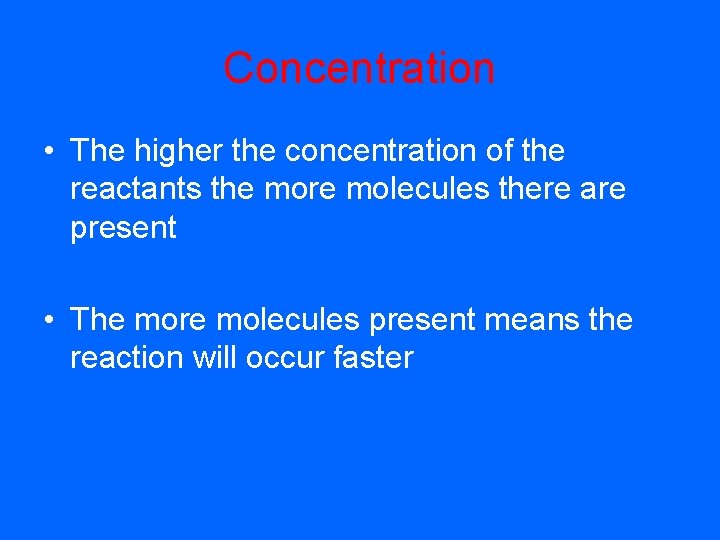
Concentration • The higher the concentration of the reactants the more molecules there are present • The more molecules present means the reaction will occur faster

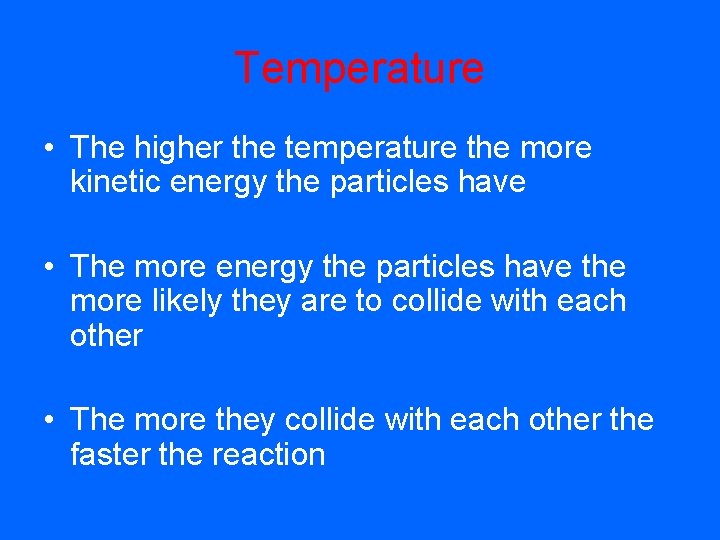
Temperature • The higher the temperature the more kinetic energy the particles have • The more energy the particles have the more likely they are to collide with each other • The more they collide with each other the faster the reaction

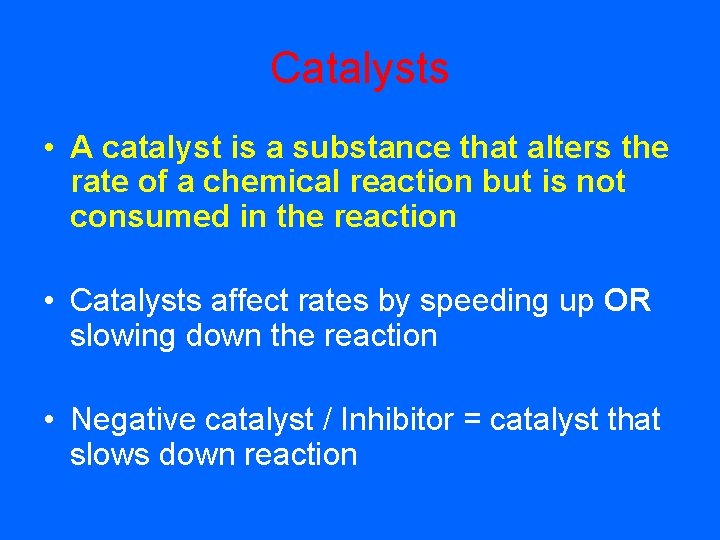
Catalysts • A catalyst is a substance that alters the rate of a chemical reaction but is not consumed in the reaction • Catalysts affect rates by speeding up OR slowing down the reaction • Negative catalyst / Inhibitor = catalyst that slows down reaction

• 5 general properties: 1. recovered unchanged at the end of the reaction 2. specific for a reaction – not all catalysts work for every reaction 3. only need a small amount

4. help equilibrium to be reached faster in equilibrium reactions 5. the action of a catalyst can be destroyed by a catalytic poison

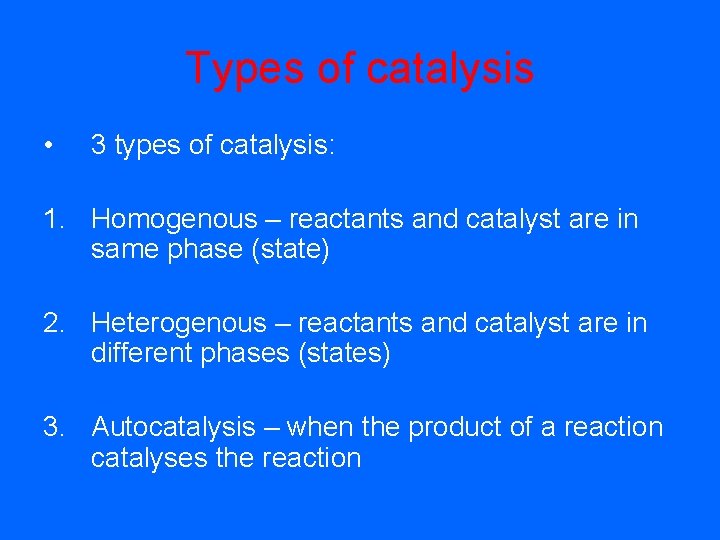
Types of catalysis • 3 types of catalysis: 1. Homogenous – reactants and catalyst are in same phase (state) 2. Heterogenous – reactants and catalyst are in different phases (states) 3. Autocatalysis – when the product of a reaction catalyses the reaction

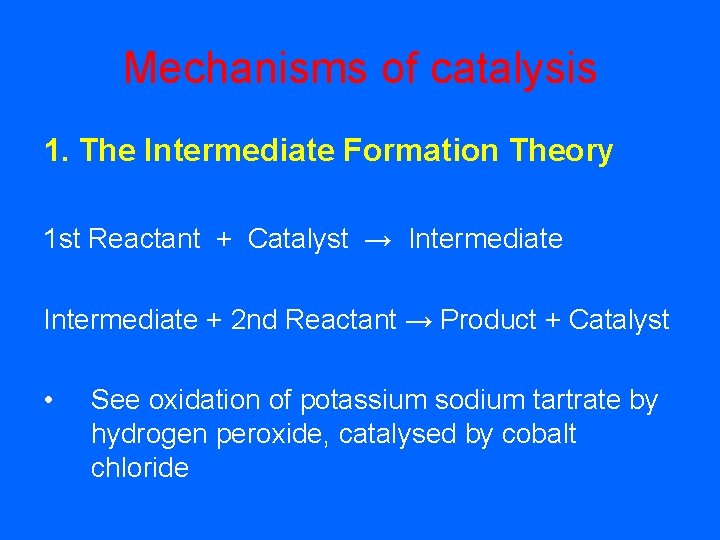
Mechanisms of catalysis 1. The Intermediate Formation Theory 1 st Reactant + Catalyst → Intermediate + 2 nd Reactant → Product + Catalyst • See oxidation of potassium sodium tartrate by hydrogen peroxide, catalysed by cobalt chloride

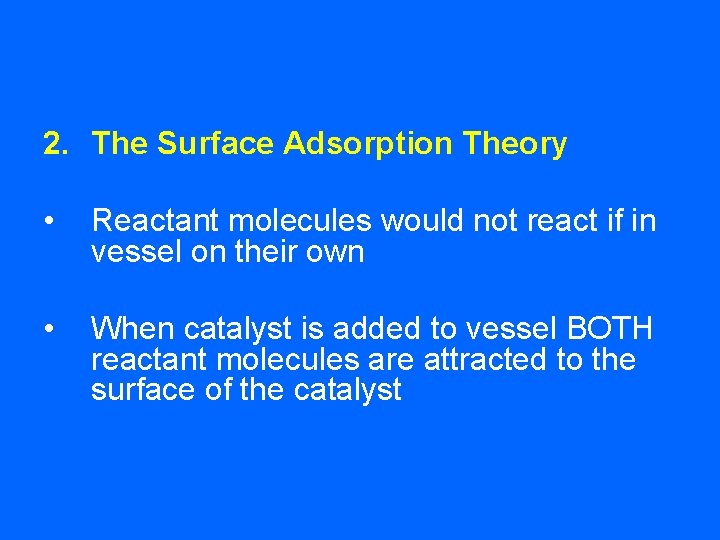
2. The Surface Adsorption Theory • Reactant molecules would not react if in vessel on their own • When catalyst is added to vessel BOTH reactant molecules are attracted to the surface of the catalyst

• When they meet at the surface of the catalyst they are close enough together to react and form product molecules • These product molecules then leave the surface of the catalyst making room for more reactant molecules to adsorb onto it

Catalytic converters • Exhaust fumes are a major cause of pollution • They contain harmful gases like CO, NO 2 and unburned hydrocarbons • We need to reduce these harmful emissions

• A catalytic converter is attached to the cars exhaust system to convert the harmful gases to harmless ones • Converter consists of a ceramic or metal honeycomb coated in palladium (Pd), rhodium (Rh) or platinum (Pt)

• The honeycomb has a huge surface area so lots of reactions can occur • A mixture of hot harmful gases passes through the honeycomb where surface adsorption occurs converting the gases into harmless ones • Unburned hydrocarbons, CO, NO and NO 2 are converted to harmless gases like H 2 O, CO 2 and N 2

• 2 CO + 2 NO N 2 + 2 CO 2 • The catalysts can be recycled because they are not consumed in the reactions • Decay is caused by poisoning or disintegration of catalysts &disintegration of honeycomb

Collision Theory • Based on the Kinetic Theory of Gases • For a reaction to occur collisions must occur • Products are only formed if a certain minimum energy is exceeded producing an effective collision

• When effective collisions occur bonds are broken and new ones are formed • According to the Collision Theory the rate of reaction depends on the number of collisions per second AND the number of effective collisions

Activation Energy • For effective collisions to occur a certain energy is needed • Activation energy is the minimum energy that colliding particles must have for a reaction to occur • The size of the Activation Energy depends on the nature of the reactants

• LOW activation energy means a FAST reaction because the molecules are more likely to have enough energy for effective collisions • HIGH activation energy means a SLOW reaction because the molecules are less likely to have sufficient energy for effective collisions

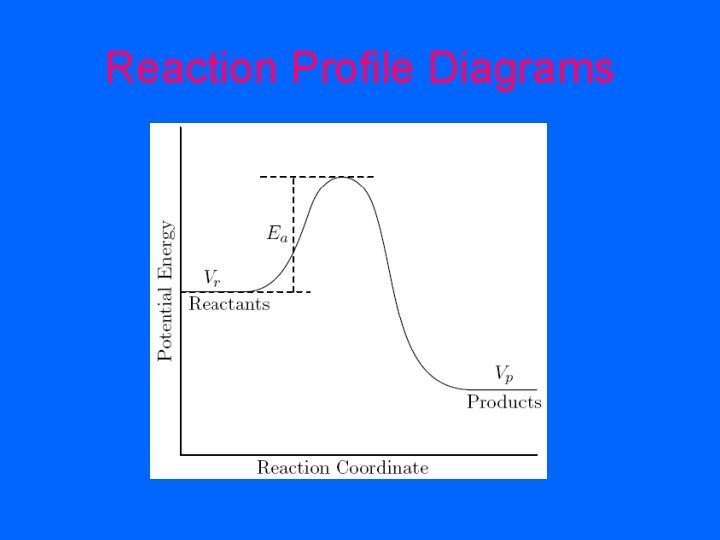
Reaction Profile Diagrams

• Exothermic reaction: - energy of the products is lower than the reactants - the difference in energy is called ΔH and is negative because energy is released as heat

• Endothermic reaction: - the energy of the products is higher than the energy of the reactants - the difference in energy, ΔH, is positive because energy from the environment has been absorbed by the reaction

• Effect of catalyst on Activation Energy: - lowers the activation energy - means more collision will be effective collisions so the products will be formed quicker - so increases the rate

Collision Theory and Factors Effecting Rates 1) Nature of reactants: • Reactions involving bond breaking require energy so have a much higher activation energy and therefore a slower rate of reaction

2) Particle size: • The smaller the particle size the greater the surface area so more collisions can occur • More collisions means more effective collisions • More effective collisions means a faster rate of reaction

3) Concentration: • If the concentration of reactants is increased more reactant molecules are present • More reactant molecules means more collisions can occur • More collisions means more effective collisions • More effective collisions means a faster rate of reaction .

4) Temperature: • The higher the temperature the more kinetic energy the reactant molecules have • The more energy they have the more likely they are to collide • More collisions means more effective collisions • More effective collisions means a faster rate of reaction

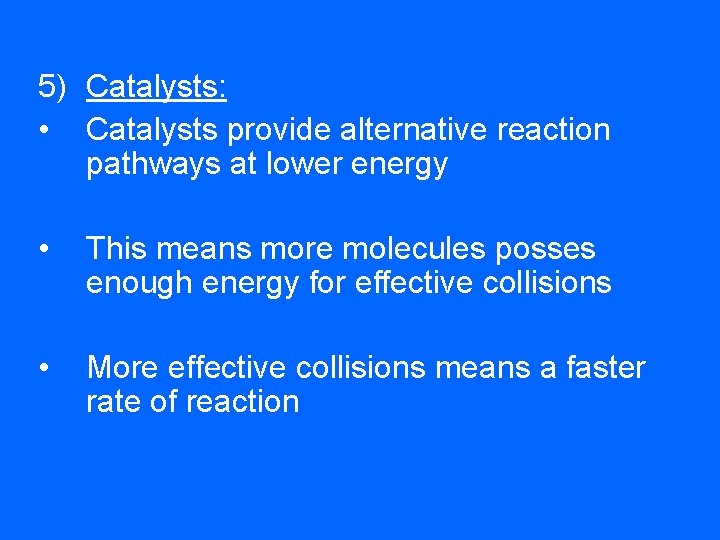
5) Catalysts: • Catalysts provide alternative reaction pathways at lower energy • This means more molecules posses enough energy for effective collisions • More effective collisions means a faster rate of reaction

Explosions • In industry dust explosions can occur due to particle size • If you have small, dry, combustible particles in the presence of oxygen and there is a source of ignition then an explosion can occur

Experiments: Curved graphs: • When you get a curve you connect all the dots • Starts off steep because there is a high concentration of reactants present • Levels off because the reaction slows down as the concentration of reactants decreases until there are no reactants left to form product

• If you increased the concentration of the reactants the graph would start off steeper and level off later as more product would be formed • If you decreased the concentration of the reactants the graph would start off less steep and would level off sooner because less product would be formed

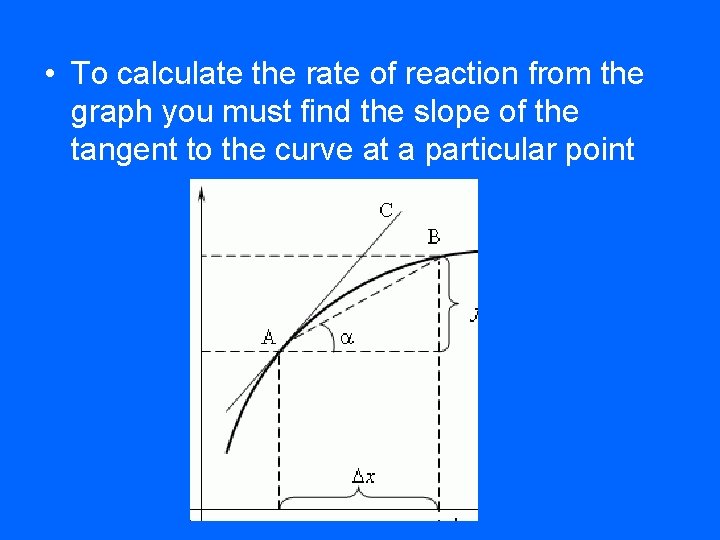
• To calculate the rate of reaction from the graph you must find the slope of the tangent to the curve at a particular point

Straight line graphs: • You may have to work out the rate: rate α 1 t • Should always get a straight fit line through the origin • Straight fit line means that the concentration/temperature is directly proportional to the rate
 Rates of reaction can be positive or negative. true false
Rates of reaction can be positive or negative. true false Two opposing processes occur at equal rates
Two opposing processes occur at equal rates Is a ratio a rate
Is a ratio a rate Ratios and proportions guided notes
Ratios and proportions guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Reduction half reaction
Reduction half reaction Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Where is the mitochondria located
Where is the mitochondria located Did a chemical reaction occur
Did a chemical reaction occur Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Expressing reaction rates
Expressing reaction rates B and q candles
B and q candles Social engineering can occur through
Social engineering can occur through Effortful processing can occur only with
Effortful processing can occur only with Statement level concurrency
Statement level concurrency Mechanics of heat transfer
Mechanics of heat transfer Types of chemical reactions redox
Types of chemical reactions redox Different types of reactions
Different types of reactions Reaction rate equation
Reaction rate equation E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Thermosoftening plastics examples
Thermosoftening plastics examples Technicolor test
Technicolor test Sound will travel at different speeds in different mediums.
Sound will travel at different speeds in different mediums. Sound will travel at different speeds in different mediums.
Sound will travel at different speeds in different mediums. Cultural relativism
Cultural relativism