Properties of Water Water is ESSENTIAL to life










- Slides: 19

Properties of Water

Water is ESSENTIAL to life. Living organisms are composed mostly of water. Humans – 65% Jellyfish – 90% Tomato – 90% Elephant – 70%

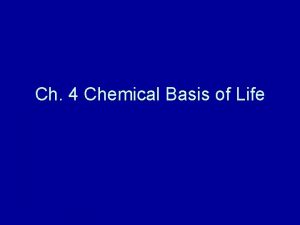
• H atoms are “attached” to one side of the O atom • This results in water molecule having a (+) charge on the side where the H atoms are and a (–) charge on the side where oxygen is. • This uneven distribution of charge is called polarity.

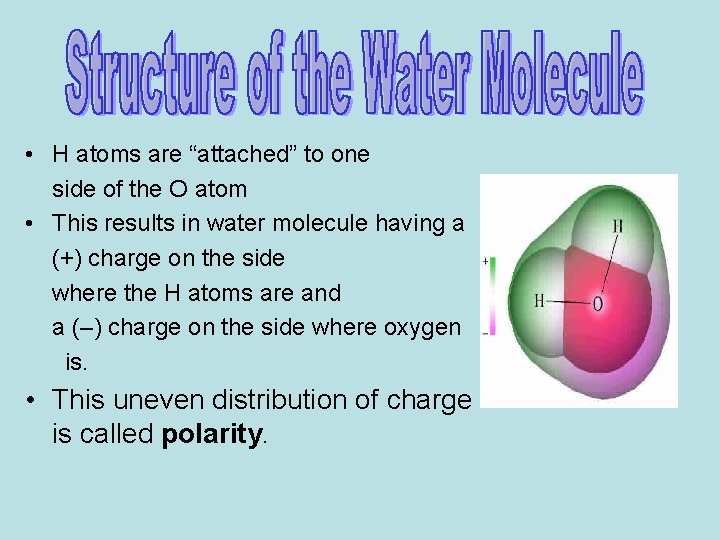
• Opposite electrical charges attract • Water molecules tend to attract each other – Side with H atoms (+) attracts O side (-) of a different water molecule

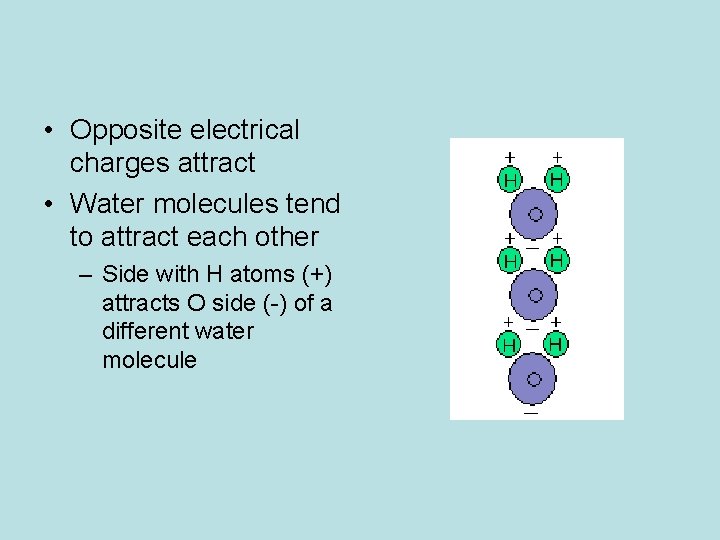
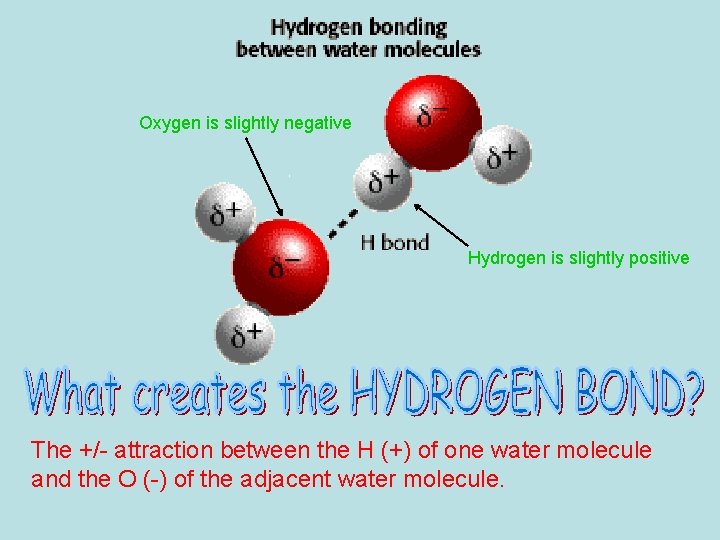
Oxygen is slightly negative Hydrogen is slightly positive The +/- attraction between the H (+) of one water molecule and the O (-) of the adjacent water molecule.

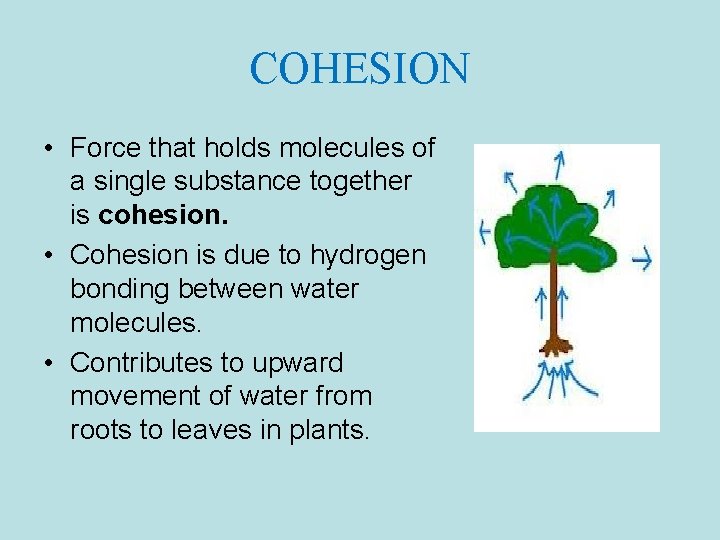
COHESION • Force that holds molecules of a single substance together is cohesion. • Cohesion is due to hydrogen bonding between water molecules. • Contributes to upward movement of water from roots to leaves in plants.

SURFACE TENSION • Related to cohesion because water molecules are attracted to other water molecules. • Water molecules at the surface are pulled into body of water which causes it to bead up.

• Because of surface tension, water holds its shape and will not spread out • For water to spread out, surface tension must be reduced – Chemicals such as surfactants can reduce the surface tension • Surface tension enables water-striders to run on water without breaking the surface

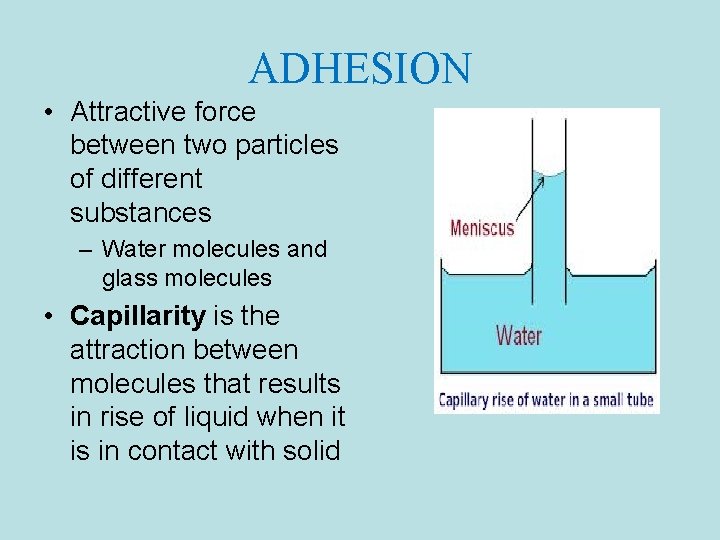
ADHESION • Attractive force between two particles of different substances – Water molecules and glass molecules • Capillarity is the attraction between molecules that results in rise of liquid when it is in contact with solid

Temperature Moderation • Water can absorb or release large amounts of energy in the form of heat with only a slight change in temperature. – known as Heat Capacity • Another result of the multiple H bonds • A large amount of heat energy is needed for movement of water molecules to increases the temperature

• Hot summer day – water can absorb large amount of energy from sun and can cool the air without large increase in water temp. • At night – gradual cooling water warms the air

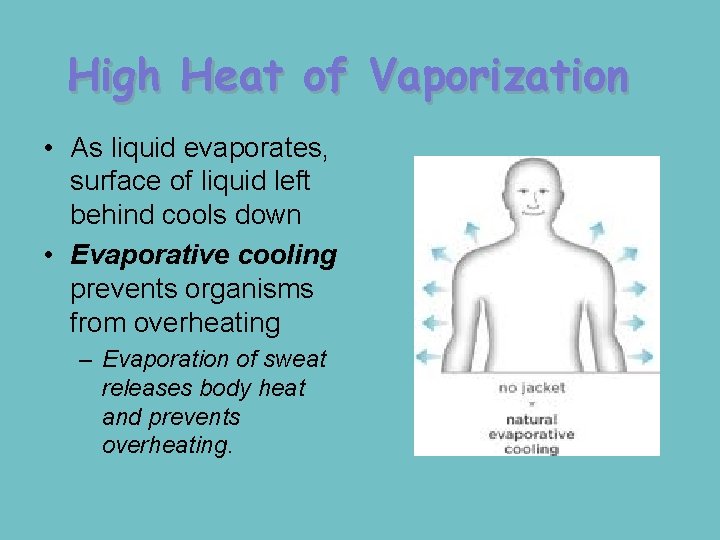
High Heat of Vaporization • As liquid evaporates, surface of liquid left behind cools down • Evaporative cooling prevents organisms from overheating – Evaporation of sweat releases body heat and prevents overheating.

Water is Less Dense as a Solid • Which is ice and which is water?

Water is Less Dense as a Solid • Ice is less dense as a solid than as a liquid (ice floats) • Liquid water has hydrogen bonds that are constantly being broken and reformed. • Frozen water forms a crystal-like lattice whereby molecules are set at fixed distances.

Water is Less Dense as a Solid Water Ice

Mixtures • Composed of two or more elements or compounds that are physically mixed together but not chemically combined • Water can make 2 types of mixtures: solutions & suspensions • Suspension: mixtures of water and nondissolved material • Biological example: blood

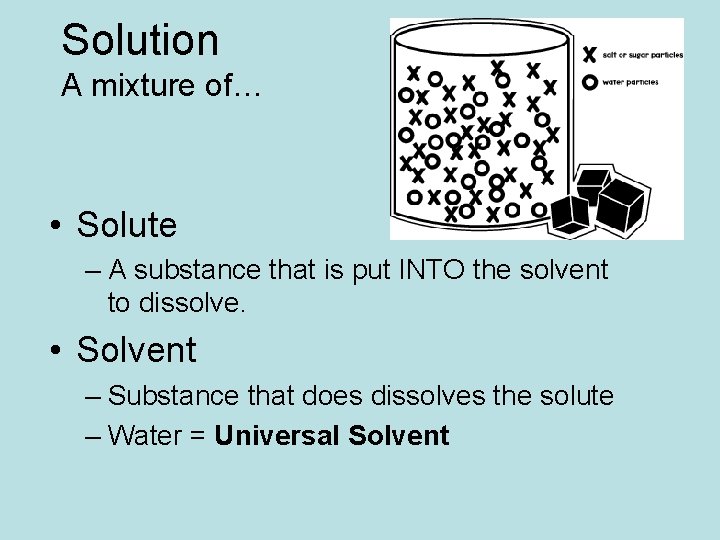
Solution A mixture of… • Solute – A substance that is put INTO the solvent to dissolve. • Solvent – Substance that does dissolves the solute – Water = Universal Solvent

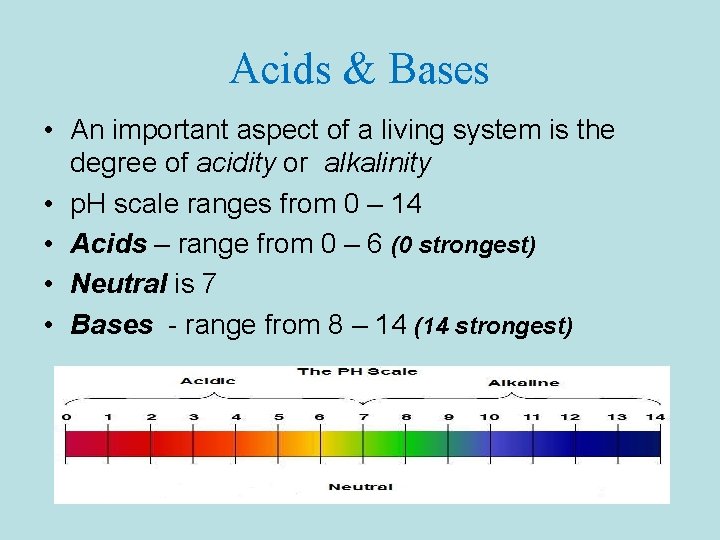
Acids & Bases • An important aspect of a living system is the degree of acidity or alkalinity • p. H scale ranges from 0 – 14 • Acids – range from 0 – 6 (0 strongest) • Neutral is 7 • Bases - range from 8 – 14 (14 strongest)

Acids, Bases and Buffers • H 2 O H+ + OH _ • Water dissociated into equal amounts of hydrogen ions and hydroxide ions • The p. H of pure water is 7 (neutral) • Acids have more hydrogen ions than hydroxide ions • Bases have more hydroxide ions than hydrogen ions
 Water and water and water water
Water and water and water water Characteristics of lipids
Characteristics of lipids What are the life supporting properties of water
What are the life supporting properties of water 25 elements essential to life
25 elements essential to life Water cycle essential questions
Water cycle essential questions Extensive and intensive examples
Extensive and intensive examples Chemical property definition
Chemical property definition Water concept map
Water concept map Properties of water ap bio
Properties of water ap bio Physical properties of seawater
Physical properties of seawater Properties of water clipart
Properties of water clipart Properties of water lab
Properties of water lab Ocean water properties
Ocean water properties Propeties of water
Propeties of water Bill nye properties of water
Bill nye properties of water Honors biology properties of water lab
Honors biology properties of water lab The extraordinary properties of water
The extraordinary properties of water The extraordinary properties of water
The extraordinary properties of water Properties of water foldable
Properties of water foldable Unique facts about water
Unique facts about water