Properties of Water Properties of Water What is








- Slides: 11

Properties of Water

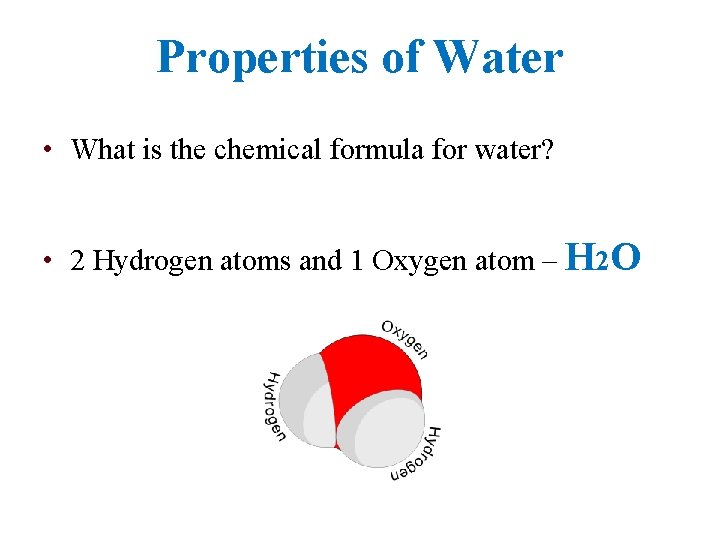
Properties of Water • What is the chemical formula for water? • 2 Hydrogen atoms and 1 Oxygen atom – H 2 O

Water • The most important molecule to living things. Why?

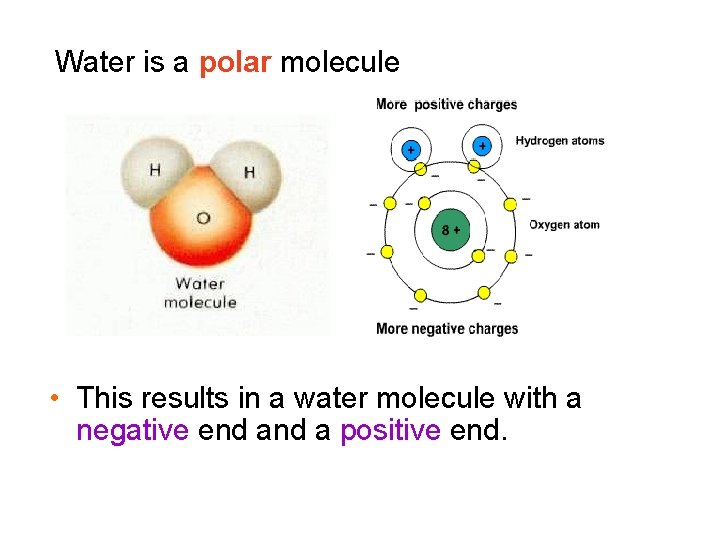
Water is a polar molecule • This results in a water molecule with a negative end a positive end.

Because water is polar it dissolves many substances. • Water is considered the Universal Solvent, meaning it can dissolve any polar substance.

Solubility Review • Solubility - the measure of how much solute can be dissolved by a solvent at a given temperature. • Example: Sugar dissolving in water. • Remember, the sugar dissolves faster in hot water than in cold water. • As the temperature increases, the solubility increases.

Because water is polar it has these properties: • Cohesion- water molecules stick together • Surface tension- inward pull of surface water molecules creating a smooth sheet • http: //www. youtube. com/watch? v=45 yabrnry Xk

Because water is polar it has these properties: • Adhesion - water molecules stick to other molecules • Capillary action - allows water to move UP and through materials

Specific Heat • Specific Heat - The amount of heat needed to increase the temperature by 1 degree Celsius. • Water has a HIGH Specific Heat! • It takes A LOT OF HEAT to raise the temperature of water.

Specific Heat Example • It’s a hot day! The sidewalk is hot, the sandy beach is hot. So, you decide to go to the beach. When you jump in the ocean, the water is surprisingly cool! • Why? ? ? Because it takes more heat to warm the water then the sidewalk, beach and air!

Water’s Specific Heat • Why does water have a HIGH specific heat? • It’s POLAR!!! (Of course!) • The molecules are SO attracted to each other that it takes A LOT of heat to break the molecules apart.
 Water and water and water water
Water and water and water water What is intensive in chemistry
What is intensive in chemistry Physical property and chemical property
Physical property and chemical property Hydrogen bond concept map
Hydrogen bond concept map Properties of water ap biology
Properties of water ap biology Characteristics of pure water
Characteristics of pure water Properties of water clipart
Properties of water clipart Water properties lab
Water properties lab Ocean water properties
Ocean water properties Propeties of water
Propeties of water Bill nye properties of water
Bill nye properties of water Honors biology properties of water lab
Honors biology properties of water lab




















