Unique Properties of Water Properties of Water What

















- Slides: 19

Unique Properties of Water

Properties of Water What are some characteristics that make you different from everybody else? Maybe you can play basketball really well, or have a freckle on the tip of your elbow, or maybe you can draw better than anybody else you know. It may seem strange, but each substance in the world has special characteristics that make it different from every other substance. Just like you, they have characteristics, or properties, that make them unique. Believe it or not, water has some of the most unique properties of all!

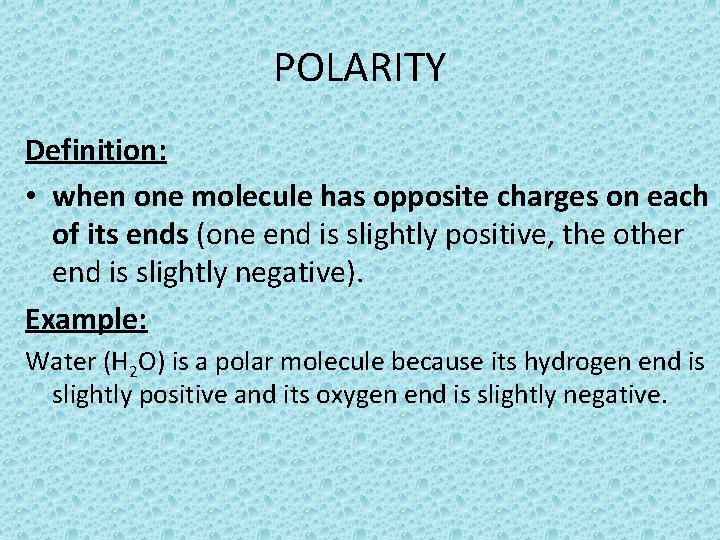
POLARITY Definition: • when one molecule has opposite charges on each of its ends (one end is slightly positive, the other end is slightly negative). Example: Water (H 2 O) is a polar molecule because its hydrogen end is slightly positive and its oxygen end is slightly negative.

ADHESION Definition: • the ability of water molecules to attract to other substances. • Because water molecules are polar (having one positive end and one negative end) they attract other substances. Example: • raindrops sticking to a window or glass • water sticking to paper towel

COHESION Definition: • the ability of water molecules to attract towards each other. It results in surface tension. – Because water molecules are polar (having one positive end and one negative end), they attract each other. Example: • rain drops

SURFACE TENSION Definition: • the force that acts on the particles at the surface of a material Example: • Water striders (bugs) • painful belly flop

DENSITY Definition: • the measure of mass of a substance per unit volume Example: • ice floating on water

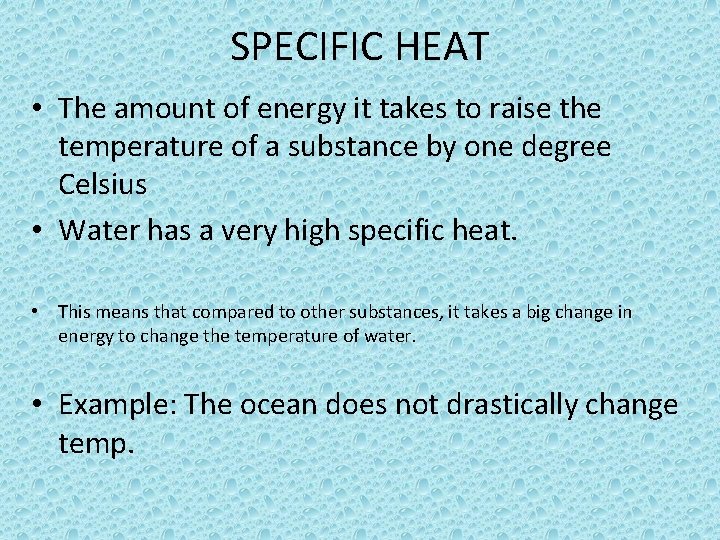
SPECIFIC HEAT • The amount of energy it takes to raise the temperature of a substance by one degree Celsius • Water has a very high specific heat. • This means that compared to other substances, it takes a big change in energy to change the temperature of water. • Example: The ocean does not drastically change temp.

UNIVERSAL SOLVENT • Water is called the “universal solvent” Definition: • this means that water can dissolve more things than any other substance. Example: • water can dissolve: soap, sugar, salt, toothpaste, baking soda, etc. Solute- substance that is being dissolved Solvent- substance into which the solute dissolves

Assessment Statements 1. The water is sticking to the side of the glass. adhesion 2. Water droplets combine together in the atmosphere to make rain drops. cohesion 3. Salt dissolves in water. universal solvent 4. When I dropped a rock in the river, it sank. buoyancy 5. On the coast in the summer, the ocean takes a lot longer to heat up than the air and land. specific heat 6. Water molecules have a positive end a negative end. polarity

Water Properties Practice 1. 2. The suction cup will only stick to the window if I wet it first. My mom used water to make Gatorade for the team. All of the powder dissolved completely in water. 3. Water striders are able to walk on water without sinking. 4. I tried to mix the oil with the water, but no matter what I did the two liquids would not mix. 5. The window was covered in rain. As the rain ran down the window, the drops came together to make larger drops of water. 6. Gina’s doctor suggested that she join a water aerobics class for exercise to keep from re-injuring her knee. He told her that the water takes the pressure off of her knee. 7. The paper towel soaked up the spill in a jiffy. 8. The log floated down the river. 9. The blue dye in the water traveled up to the petals of the white flower. 10. An iceberg floats on water in the Artic.

Unique Properties of Water Mini Labs 1. 2. 3. 4. 5. Sink’n Lincoln Water bug Water Rope Iceberg Shipwreck

Sink’n Lincoln • Predict how many drops of water you can fit on a penny. • What was the actual number of drops you could fit on the penny? • What property of water allowed you to fit that many drops on the penny?

Water bug 4. A paper clip is not lighter than water, yet it can stay on top of the water. Explain why this is. 5. Explain what the soap did to the surface tension of the water.

Water Rope 6. What is capillary action? How does this help plants?

Iceberg 7. Why does ice float on top of water? 8. Rank the densities of the liquids from most dense to least dense. 9. If water’s density is 1. 0 g/m. L, what can be determined about the other liquids’ densities in the column?

Ship Wreck 10. Does the density of the boat affect the buoyancy?

How Stuff Works: WATER 1. What is a dipole? How does water’s dipole molecular structure affect its properties? 2. Why is water essential to life on earth? Why do scientists credit water with allowing life to form? 3. How do plants transport water from the roots to the leaves? 4. How does the Hoover Dam generate electricity? What are the environmental drawbacks of damming a river? 5. Why does water resist compression? 6. What is cloud seeding? How does cloud seeding encourage rainfall?

 Water and water and water water
Water and water and water water Unique facts about water
Unique facts about water Characteristic patterns
Characteristic patterns Varian
Varian Utility coordination
Utility coordination Hobbies like tastes
Hobbies like tastes Unique nature of carbon
Unique nature of carbon Facts of the tundra
Facts of the tundra Scaffold trailer
Scaffold trailer Characteristics for saturn
Characteristics for saturn Scyphozoa characteristics
Scyphozoa characteristics Wheelaudit
Wheelaudit Unique feature of uranus
Unique feature of uranus Is bleaching your hair a physical or chemical change
Is bleaching your hair a physical or chemical change Unique pairing personality
Unique pairing personality Surface traits
Surface traits Ambiguous case example
Ambiguous case example This music is featured during sabbath and other holy days.
This music is featured during sabbath and other holy days. Unique data centers
Unique data centers E commerce 2017 business technology society
E commerce 2017 business technology society