Polymers Many Parts This name hints at how






















- Slides: 61

Polymers

Many + Parts This name hints at how polymers are made Latin: Plasticus, that which can be molded

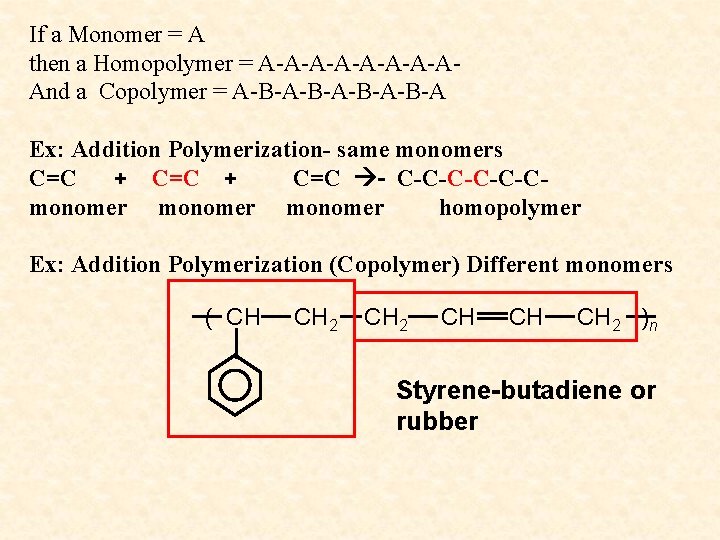
If a Monomer = A then a Homopolymer = A-A-A-A-AAnd a Copolymer = A-B-A-B-A Ex: Addition Polymerization- same monomers C=C + C=C - C-C-C-Cmonomer homopolymer Ex: Addition Polymerization (Copolymer) Different monomers ( CH CH 2 CH CH CH 2 )n Styrene-butadiene or rubber

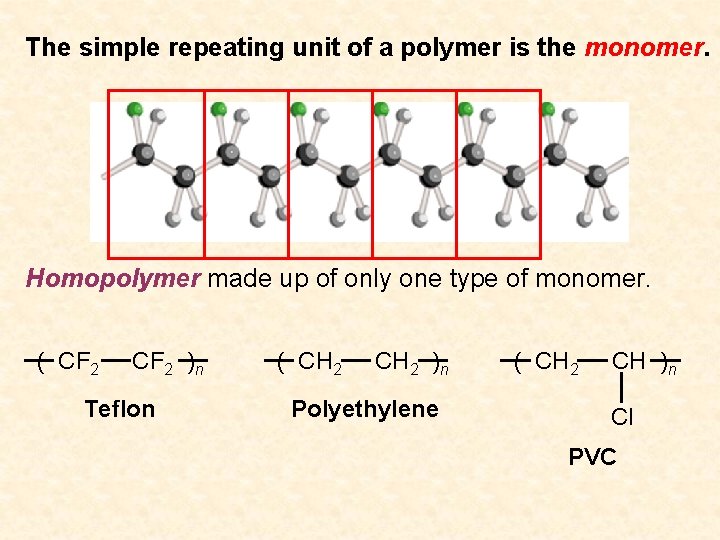
The simple repeating unit of a polymer is the monomer. Homopolymer made up of only one type of monomer. ( CF 2 )n Teflon ( CH 2 )n Polyethylene ( CH 2 CH )n Cl PVC

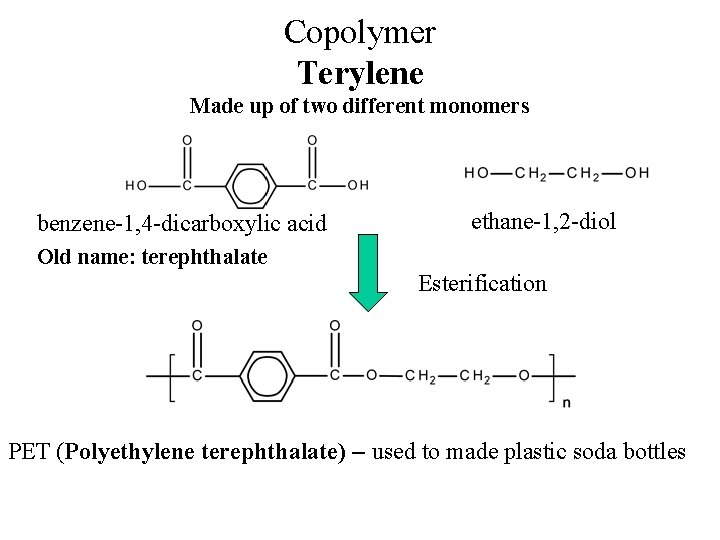
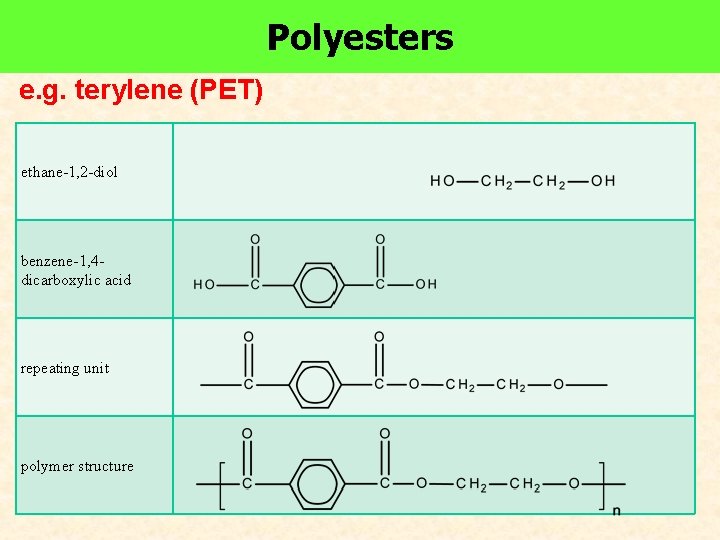
Copolymer Terylene Made up of two different monomers benzene-1, 4 -dicarboxylic acid ethane-1, 2 -diol Old name: terephthalate Esterification PET (Polyethylene terephthalate) – used to made plastic soda bottles

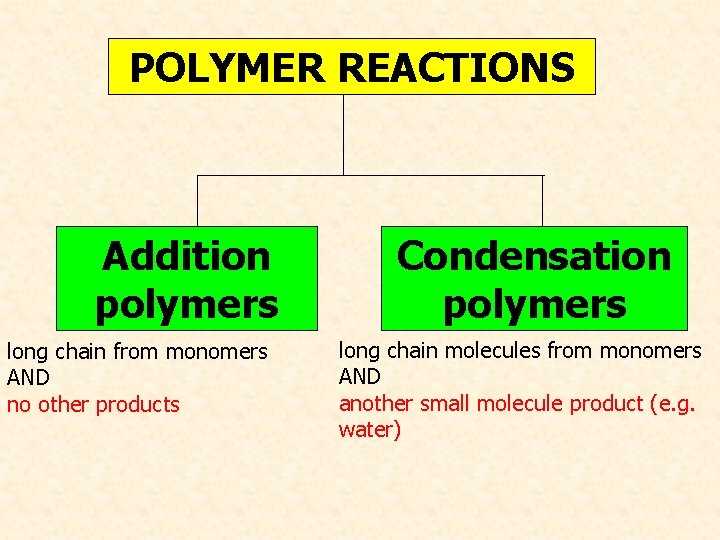
POLYMER REACTIONS Addition polymers long chain from monomers AND no other products Condensation polymers long chain molecules from monomers AND another small molecule product (e. g. water)

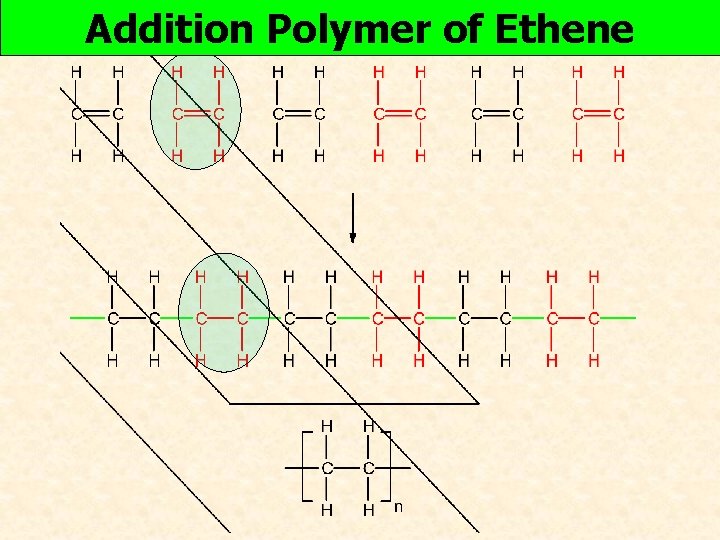
Addition Polymer of Ethene

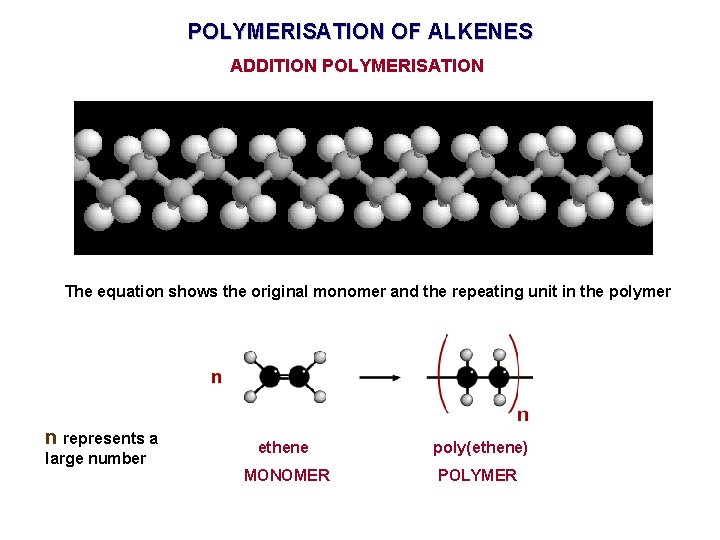
POLYMERISATION OF ALKENES ADDITION POLYMERISATION The equation shows the original monomer and the repeating unit in the polymer n represents a large number ethene poly(ethene) MONOMER POLYMER

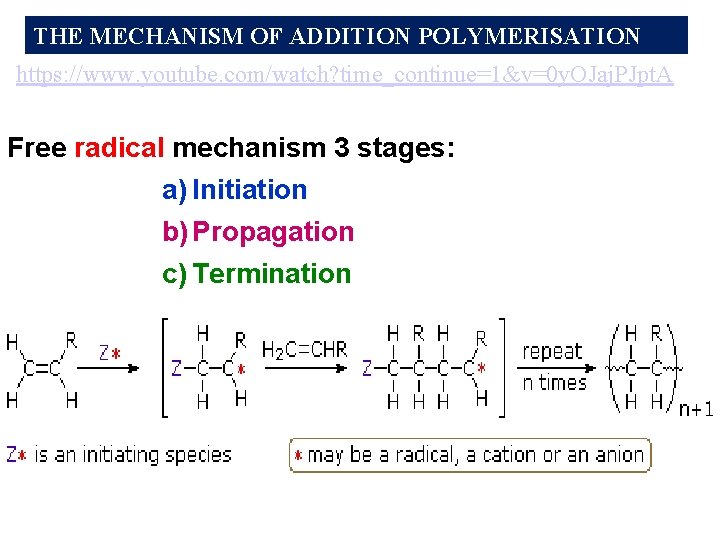
Free Radical Polymerization (3 minute clip) https: //www. youtube. com/watch? v=0 y. OJaj. PJpt. A

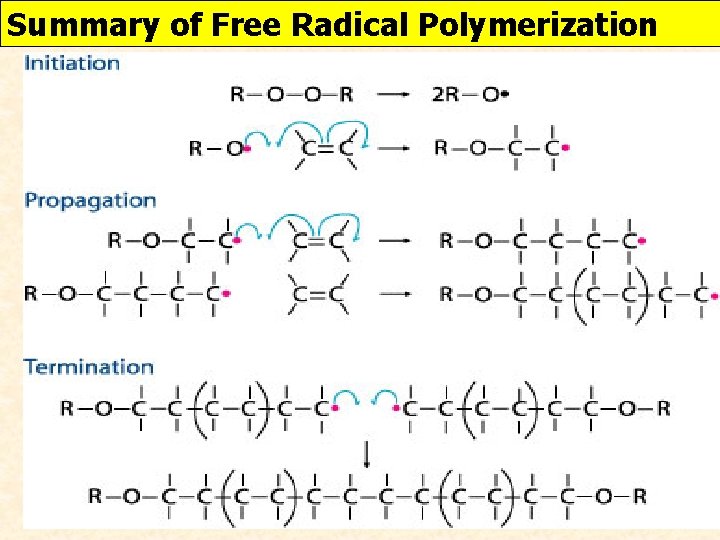
THE MECHANISM OF ADDITION POLYMERISATION https: //www. youtube. com/watch? time_continue=1&v=0 y. OJaj. PJpt. A Free radical mechanism 3 stages: a) Initiation b) Propagation c) Termination

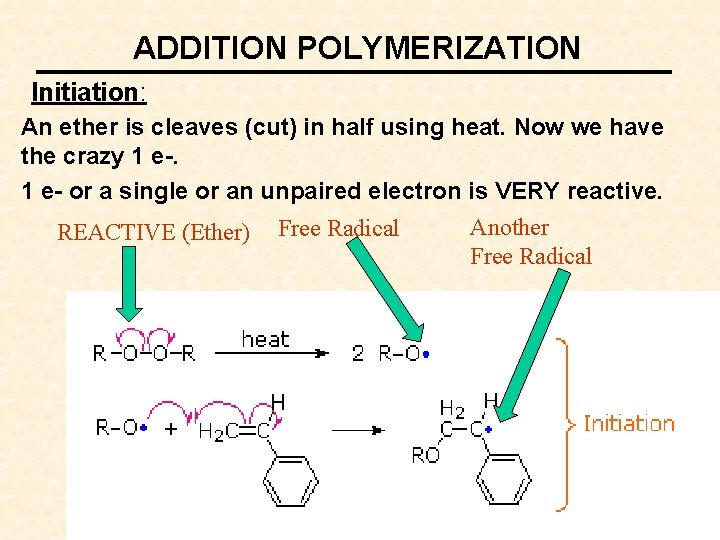
ADDITION POLYMERIZATION Initiation: An ether is cleaves (cut) in half using heat. Now we have the crazy 1 e- or a single or an unpaired electron is VERY reactive. REACTIVE (Ether) Free Radical Another Free Radical

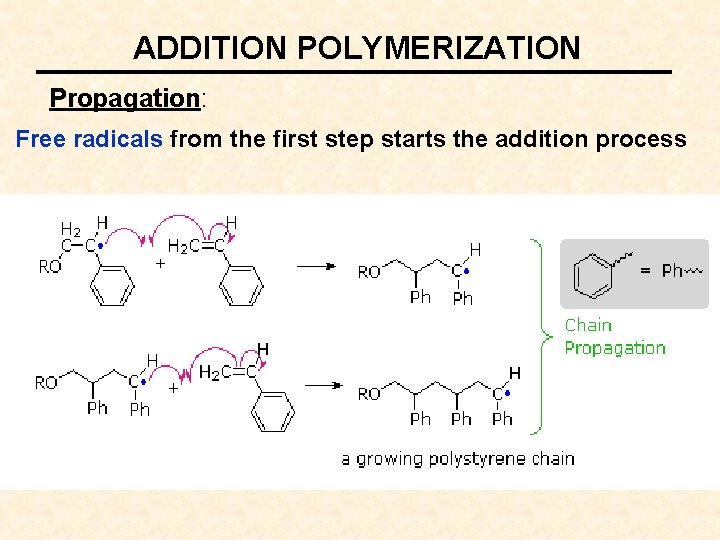
ADDITION POLYMERIZATION Propagation: Free radicals from the first step starts the addition process

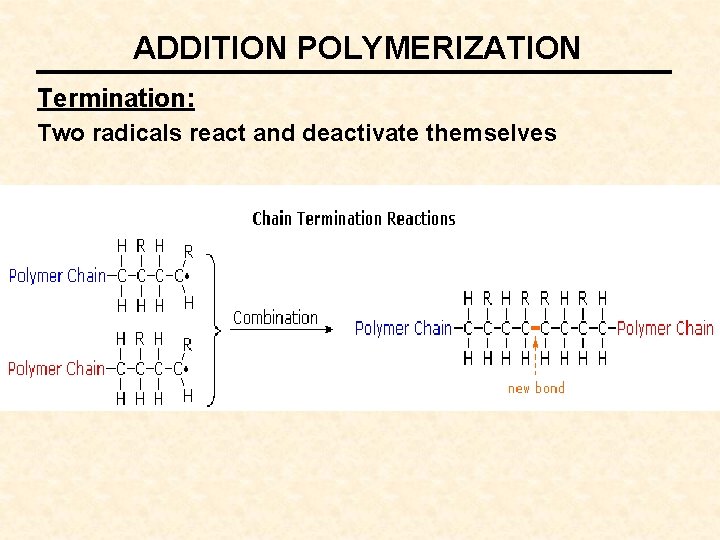
ADDITION POLYMERIZATION Termination: Two radicals react and deactivate themselves

Summary of Free Radical Polymerization

STOPPING POLYMERIZATION REACTIONS How can the polymerization reaction end or be stopped? 2 ways i) Run out or reactants (monomers) ii) 2 free radial monomers combine head to head

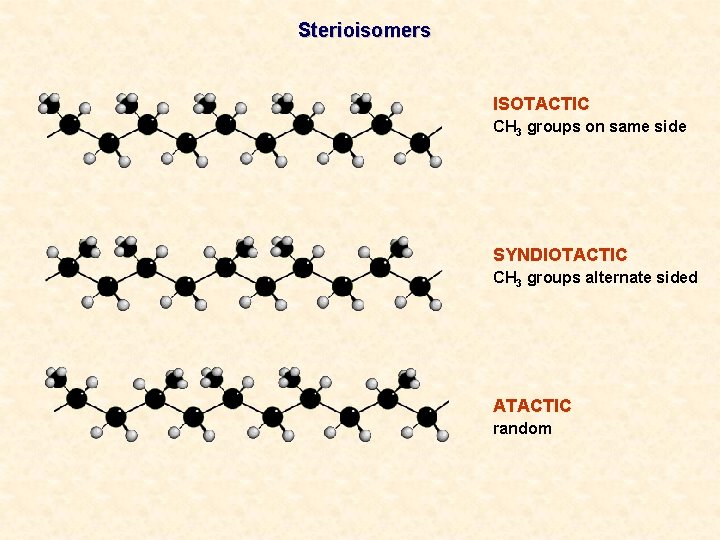
Sterioisomers ISOTACTIC CH 3 groups on same side SYNDIOTACTIC CH 3 groups alternate sided ATACTIC random

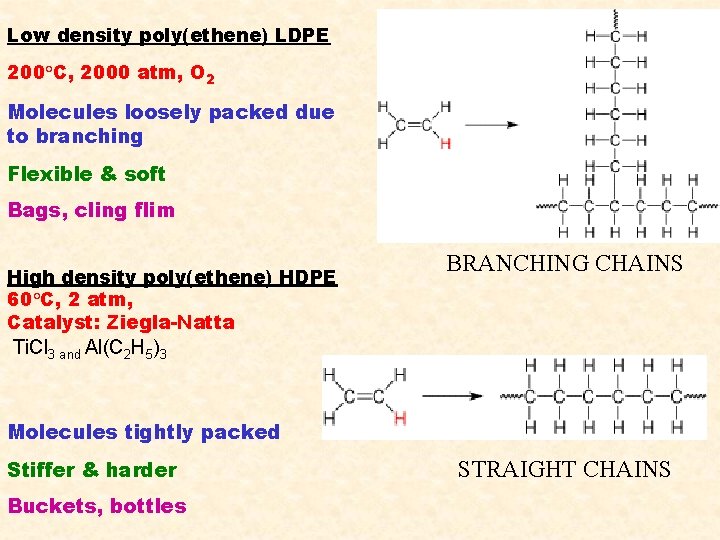
Low density poly(ethene) LDPE 200 C, 2000 atm, O 2 Molecules loosely packed due to branching Flexible & soft Bags, cling flim High density poly(ethene) HDPE 60 C, 2 atm, Catalyst: Ziegla-Natta BRANCHING CHAINS Ti. Cl 3 and Al(C 2 H 5)3 Molecules tightly packed Stiffer & harder Buckets, bottles STRAIGHT CHAINS


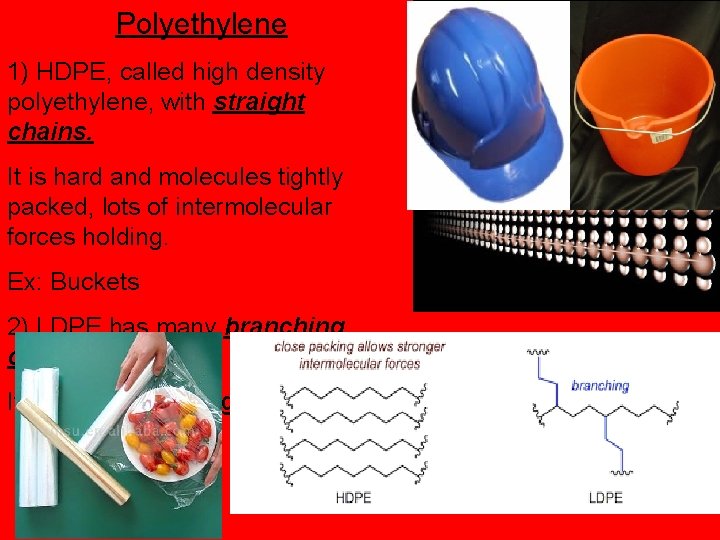
Polyethylene 1) HDPE, called high density polyethylene, with straight chains. It is hard and molecules tightly packed, lots of intermolecular forces holding. Ex: Buckets 2) LDPE has many branching chains. It is soft, Ex: “cling wrap”

Polymerisation of Ethene https: //www. youtube. com/watch? v=sk 6 h 4 oa. Ar. E 0

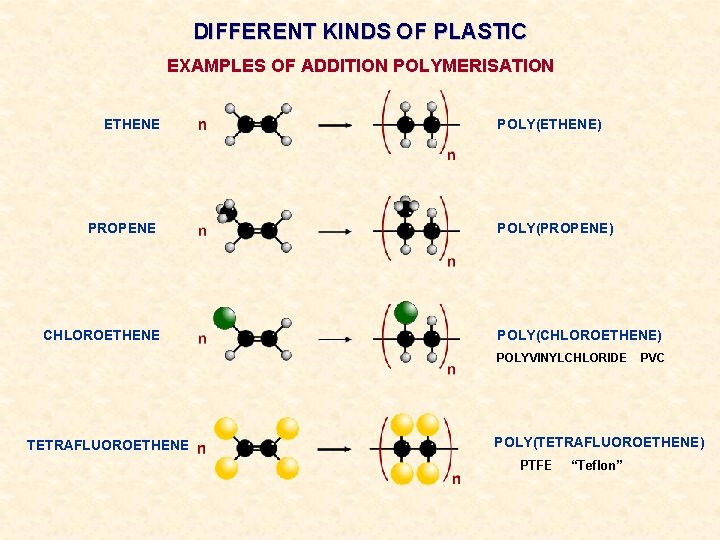
DIFFERENT KINDS OF PLASTIC EXAMPLES OF ADDITION POLYMERISATION ETHENE PROPENE CHLOROETHENE POLY(ETHENE) POLY(PROPENE) POLY(CHLOROETHENE) POLYVINYLCHLORIDE TETRAFLUOROETHENE PVC POLY(TETRAFLUOROETHENE) PTFE “Teflon”

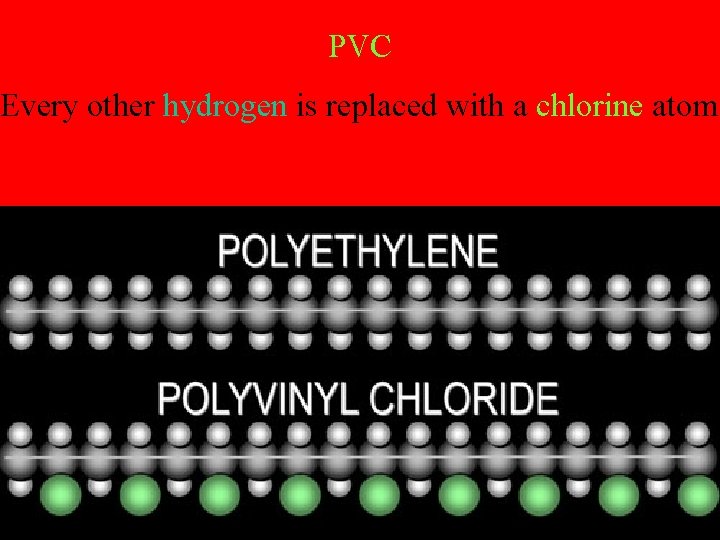
PVC Every other hydrogen is replaced with a chlorine atom

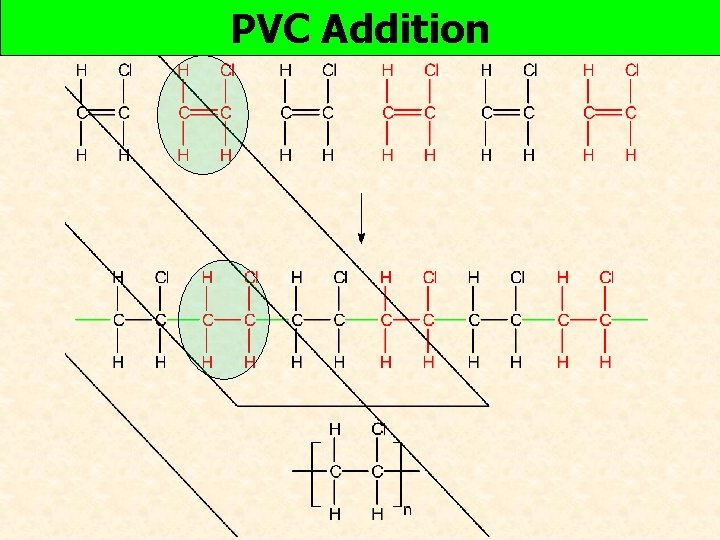
PVC Addition

PVC

Other Addition Products Ex: PTFE / Teflon

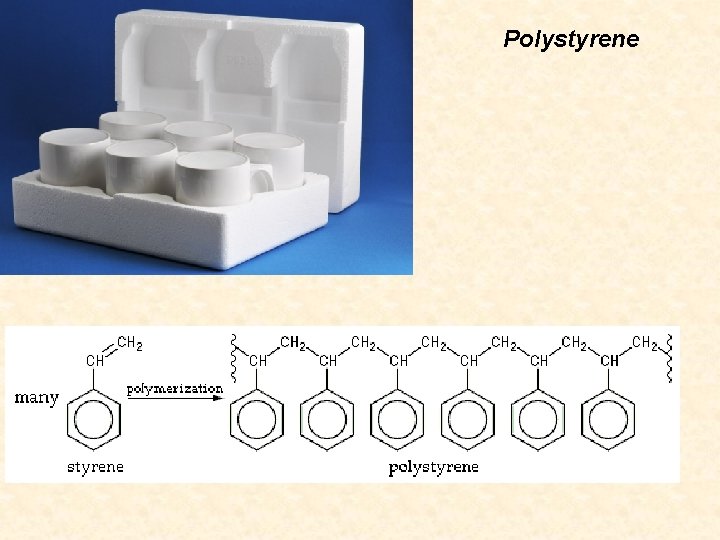
Polystyrene

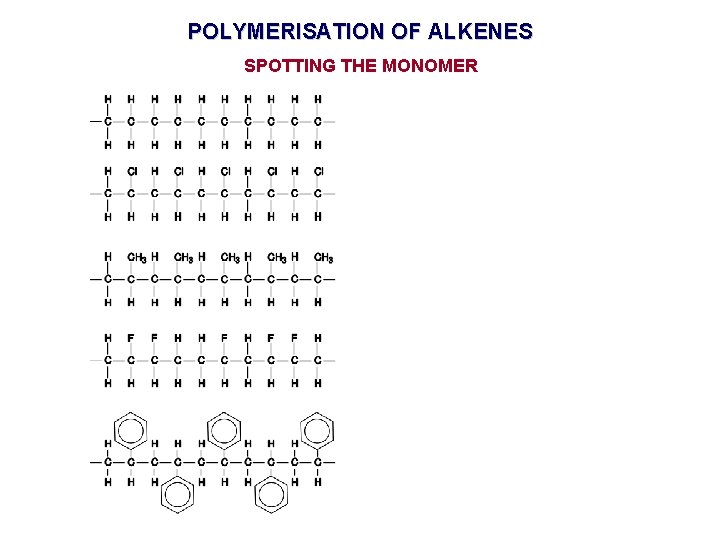
POLYMERISATION OF ALKENES SPOTTING THE MONOMER

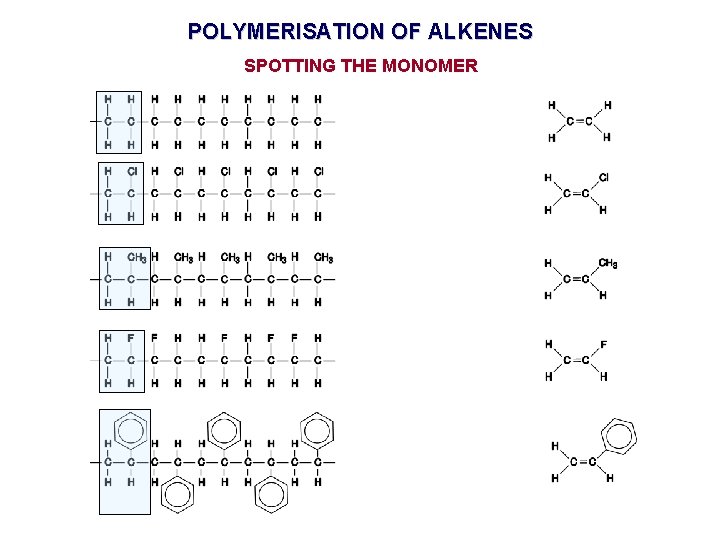
POLYMERISATION OF ALKENES SPOTTING THE MONOMER

Polymerisation of Propene https: //www. youtube. com/watch? v=nz 1 uc. I 6 g. CIg


poly(propene)

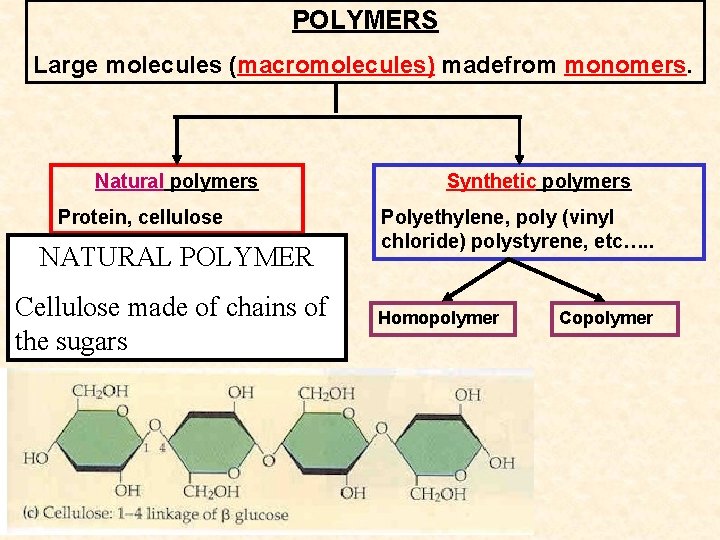
POLYMERS Large molecules (macromolecules) madefrom monomers. Natural polymers Protein, cellulose NATURAL POLYMER Cellulose made of chains of the sugars Synthetic polymers Polyethylene, poly (vinyl chloride) polystyrene, etc…. . Homopolymer Copolymer

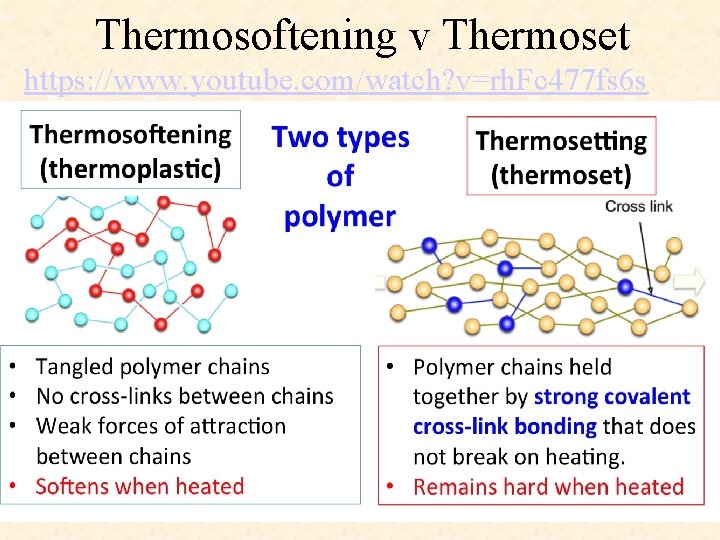
Thermosoftening v Thermoset https: //www. youtube. com/watch? v=rh. Fc 477 fs 6 s

ADDITION POLYMERISATION Chemical Properties 1)Fairly inert. Why do plastics melt but not react? The carbon–carbon covalent chemical bonds are strong so they do not break and react. But weak forces responsible for the physical properties and plastics should melt a very low temperatures, but here are many, thousands of these atoms, so plastics melt but at reasonably high temperatures Ex: 150 0 C (polyethene), like butter does. 2) Biodegradability A) Addition polymers do NOT break down B) Condensation polymers DO, why? Answer: Nu can attack the polar bonds; i. e. C-N and C-O bonds which link every polymer unit and as a result the polymers can be broken

POLYESTERS

e. g. terylene (PET)

Polyesters e. g. terylene (PET) ethane-1, 2 -diol benzene-1, 4 dicarboxylic acid repeating unit polymer structure

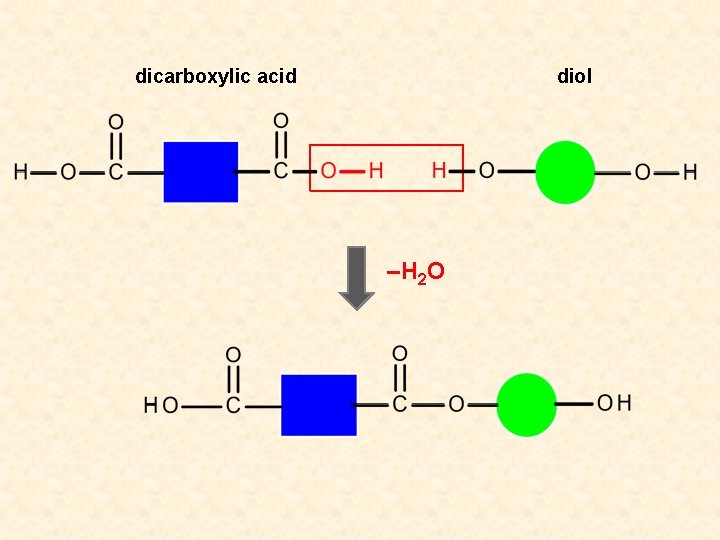
dicarboxylic acid diol –H 2 O

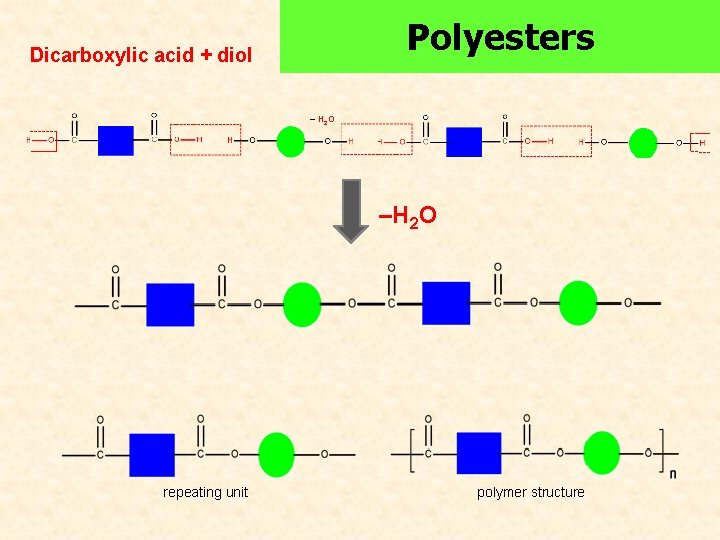
Polyesters Dicarboxylic acid + diol – H 2 O –H 2 O repeating unit polymer structure

POLYAMIDES

e. g. Kevlar

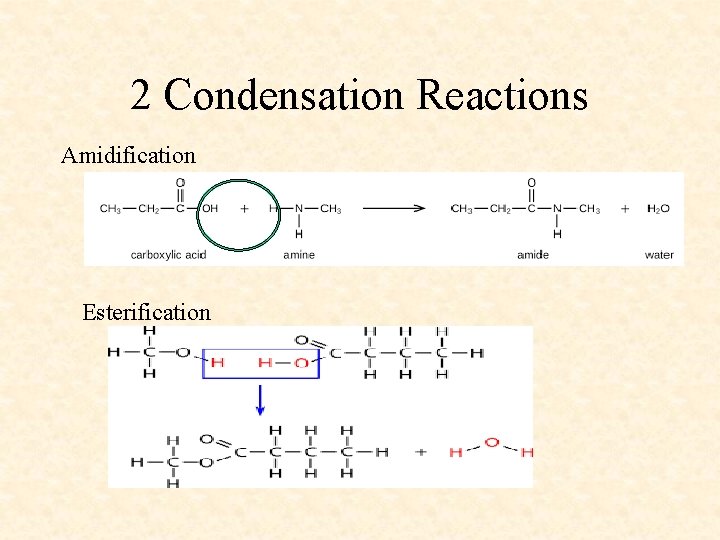
2 Condensation Reactions Amidification Esterification

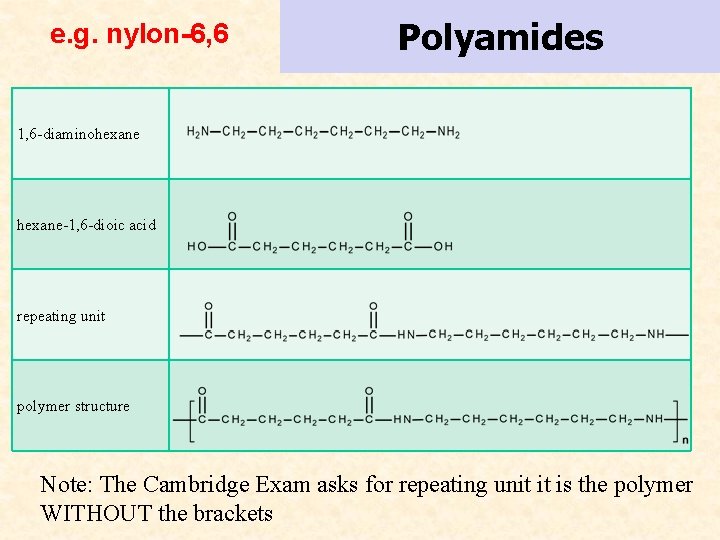
e. g. nylon-6, 6 Polyamides 1, 6 -diaminohexane-1, 6 -dioic acid repeating unit polymer structure Note: The Cambridge Exam asks for repeating unit it is the polymer WITHOUT the brackets

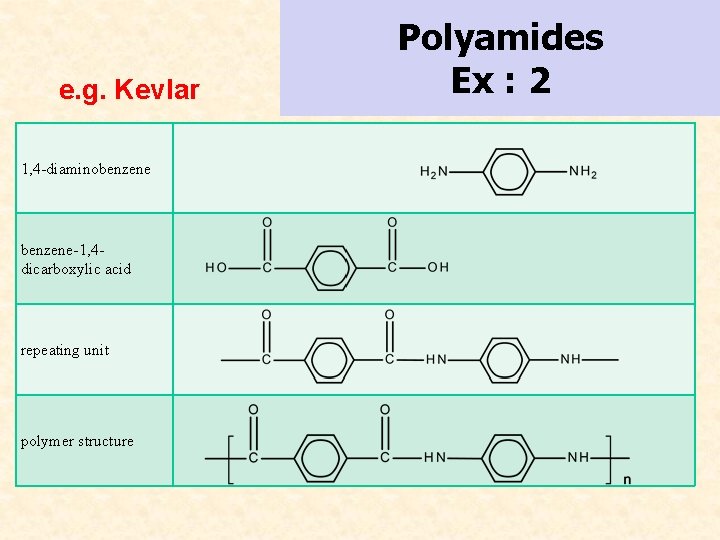
e. g. Kevlar 1, 4 -diaminobenzene-1, 4 dicarboxylic acid repeating unit polymer structure Polyamides Ex : 2

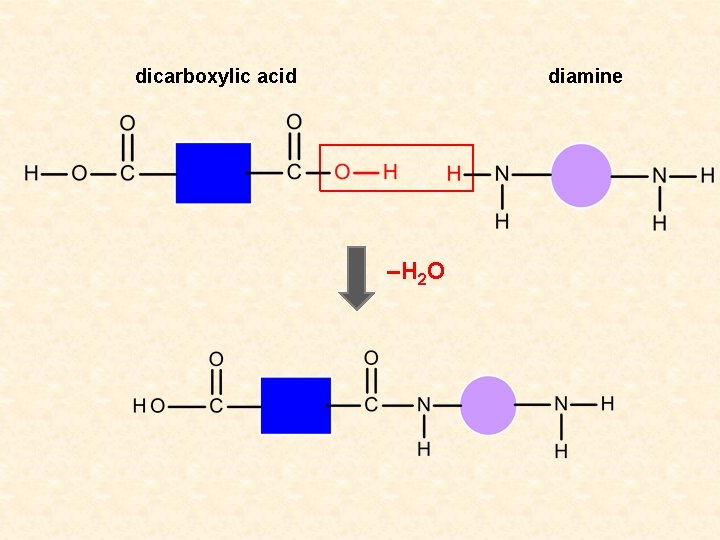
dicarboxylic acid diamine –H 2 O

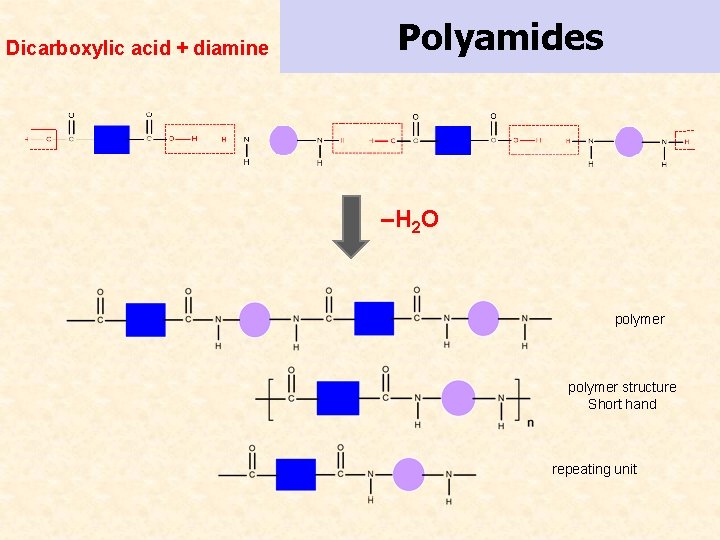
Dicarboxylic acid + diamine Polyamides –H 2 O polymer structure Short hand repeating unit

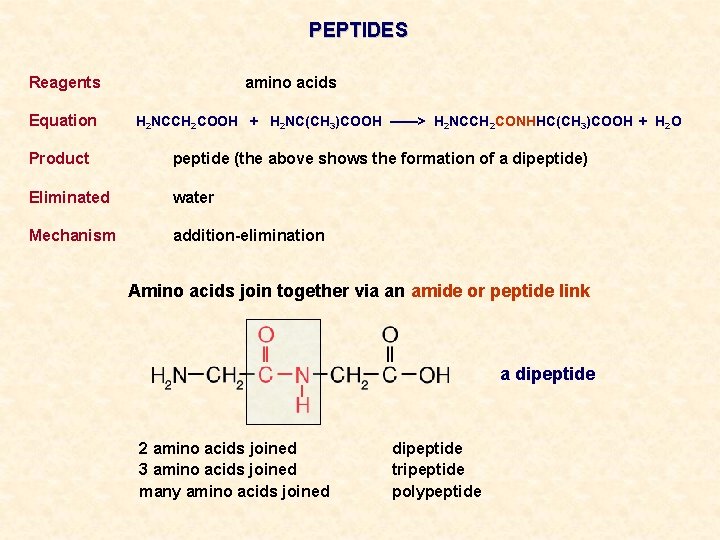
PEPTIDES Reagents Equation amino acids H 2 NCCH 2 COOH + H 2 NC(CH 3)COOH ——> H 2 NCCH 2 CONHHC(CH 3)COOH + H 2 O Product peptide (the above shows the formation of a dipeptide) Eliminated water Mechanism addition-elimination Amino acids join together via an amide or peptide link a dipeptide 2 amino acids joined 3 amino acids joined many amino acids joined dipeptide tripeptide polypeptide

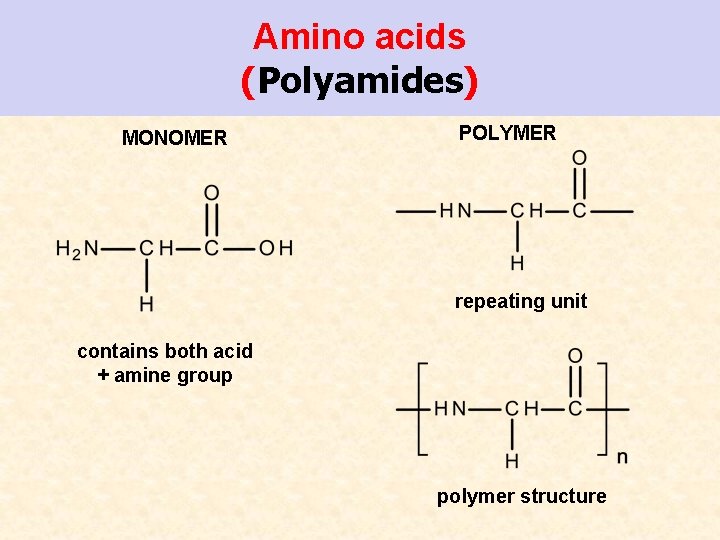
Amino acids (Polyamides) MONOMER POLYMER repeating unit contains both acid + amine group polymer structure

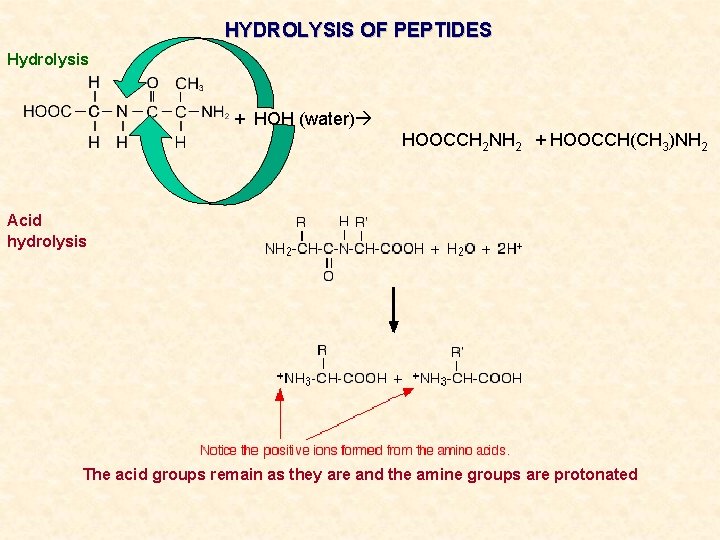
HYDROLYSIS OF PEPTIDES Hydrolysis + HOH (water) HOOCCH 2 NH 2 + HOOCCH(CH 3)NH 2 Acid hydrolysis The acid groups remain as they are and the amine groups are protonated

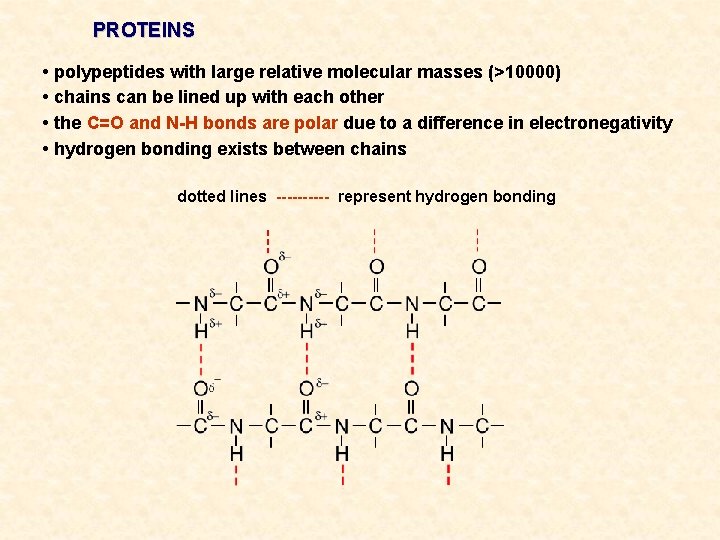
PROTEINS • polypeptides with large relative molecular masses (>10000) • chains can be lined up with each other • the C=O and N-H bonds are polar due to a difference in electronegativity • hydrogen bonding exists between chains dotted lines ----- represent hydrogen bonding

Plastic can be bad

Plastics Uses and Problems • https: //www. youtube. com/watch? v=e. Zi. BAkb. CR 0 E

Disadvantages of Plastic 1)Most plastics are non-biodegradable. 2) Plastics are made from fossil fuels which are non-renewable 3) During combustion toxic fumes are released. Combustion and toxic fumes (CH 2 CHCl)n + O 2 CO 2 + CO + HCl + H 2 O The chlorines in the PVC combine with the hydrogen atoms to form hydrogen chloride gas (HCl). When this contacts water in lungs or mouth, it turns to

Advantages of Plastics 1)They are cheap and easy to make. 2)They don’t oxidize (rust). 3)Plastics are resistant to chemical attack 4)They are easy to mould into shape and colour 5)They last a lot longer than many metals.

Biodegradable Plastics Instead of using fossil fuel derived monomers for polymerization, Starch from foods (corn starch or potato starch can be used, they can polymerize and form plastics as well as ethene or propene. Advantage: pollution control as these will rot in less than a year Disadvantage: using food when the world still has hungry people is grossly unethical. These plastics do not work as well

How much waste plastic? https: //www. youtube. com/watch? v=_6 xl. Ny. WPp. B 8 (5 minutes) There are three ways to dispose of waste plastics: l landfill l incineration (burning) l recycling Each has its own advantages and disadvantages.

Disposal of Polymers 1 Cambridge Question : Addition polymers are non-biodegradable but ccondensation polymers can degrade, why the difference? Answer : Nu can attack the polar bonds; i. e. C-N and C-O bonds which link every polymer unit and as a result the polymers can be broken Landfill Advantages ü No sorting costs ü Modern landfills do not pollute. ü Plastics break down to make methane which can generate electricity. Disadvantages ü Distance to landfill sites so transport costs. ü If too much methane builds up, explosions can occur.

Disposal of Polymers 2 Incineration (burning) Advantages Disadvantages ü Burning plastics ü Burning at low produces heat energy. temperatures can produce harmful dioxins ü Polythene produces more energy than burning coal ü Old incinerators (low or oil. temperature) produce harmful gases. ü Saves fossil fuels

Disposal of Polymers 3 Recycling Advantages ü Plastic is lightweight even when compressed. ü Cheaper to recycle plastics than make then from scratch. ü Recycled plastics can be used to make lots of useful materials ü Some plastics can be broken down to make raw materials for other products. Disadvantages ü Plastics must be collected and sorted. ü Many plastics contain materials that need to be removed. ü Cost of transporting to nearest recycling plant. ü Recycled materials are weaker

Plastics How They are Made https: //www. youtube. com/watch? v=Jarary. OXa 0 Q

Quiz http: //www. bbc. co. uk/education/guides/zxm 39 j 6/activity