Polymers Many Parts This name hints at how



















- Slides: 38

Polymers

Many + Parts This name hints at how polymers are made Latin: Plasticus, that which can be molded

Polymer • Polymers are made up of many molecules Poly- means "many" and -mer means “unit”

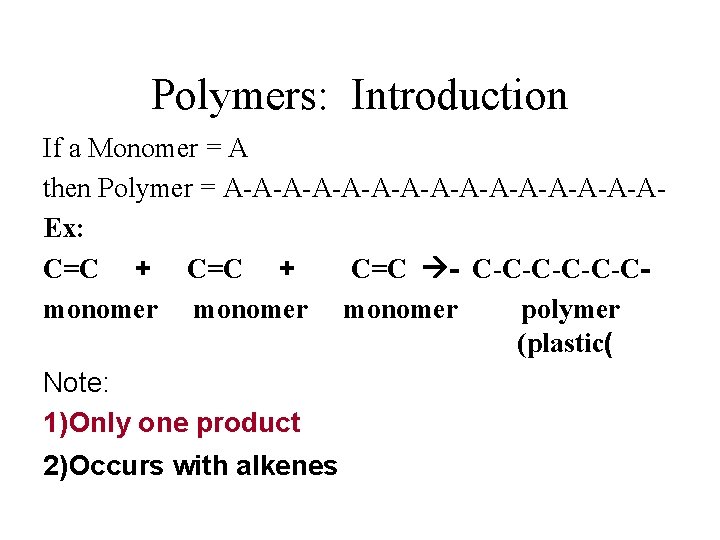
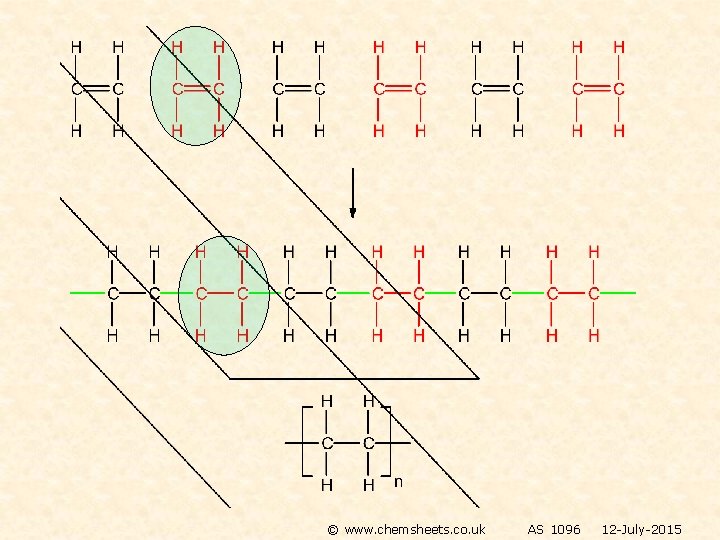
Polymers: Introduction If a Monomer = A then Polymer = A-A-A-A-A-A-A-AEx: C=C + C=C - C-C-C-Cmonomer polymer (plastic( Note: 1)Only one product 2)Occurs with alkenes

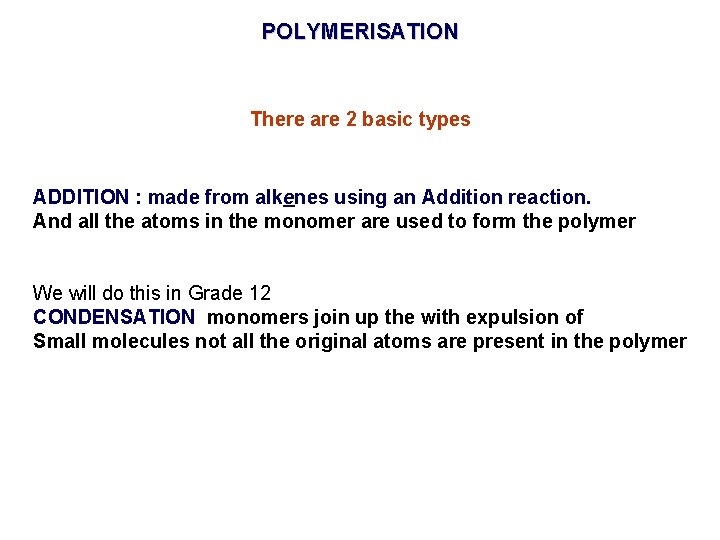
POLYMERISATION There are 2 basic types ADDITION : made from alkenes using an Addition reaction. And all the atoms in the monomer are used to form the polymer We will do this in Grade 12 CONDENSATION monomers join up the with expulsion of Small molecules not all the original atoms are present in the polymer

© www. chemsheets. co. uk AS 1096 12 -July-2015

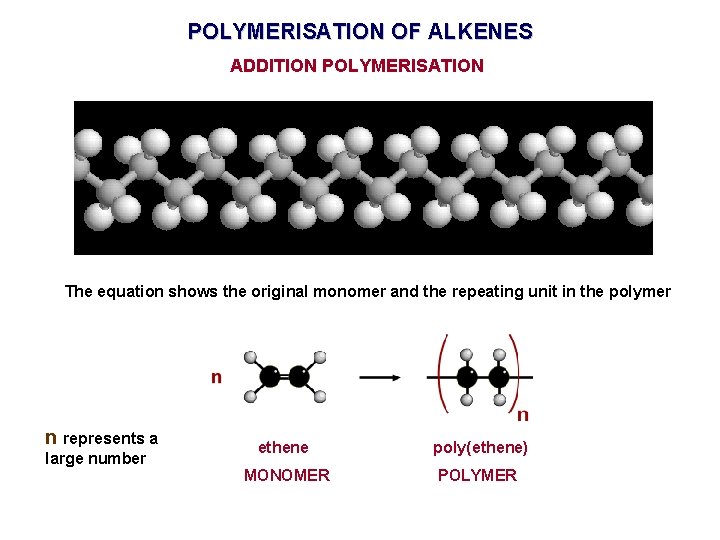
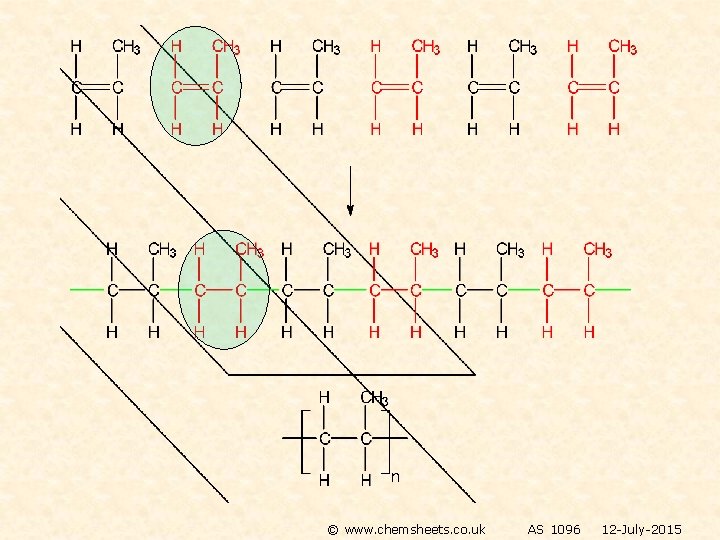
POLYMERISATION OF ALKENES ADDITION POLYMERISATION The equation shows the original monomer and the repeating unit in the polymer n represents a large number ethene poly(ethene) MONOMER POLYMER

Polyethylene. It is the most common plastic you see. It is used for bottles, buckets, jugs, containers, toys, even synthetic lumber, and many other things.

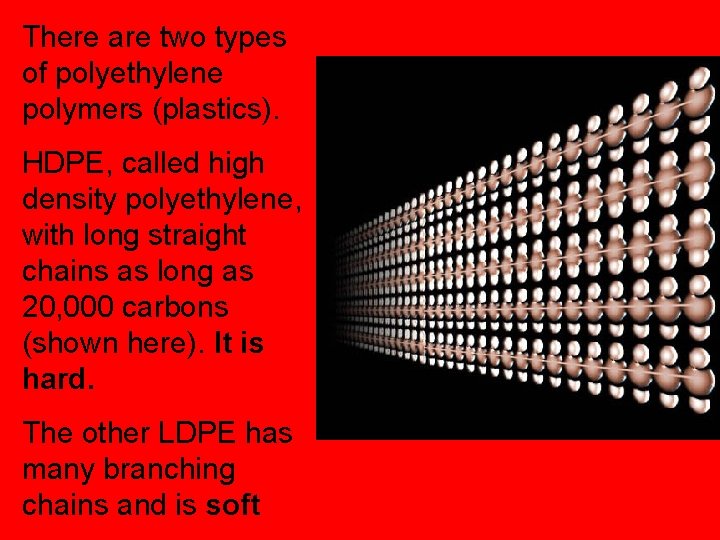
There are two types of polyethylene polymers (plastics). HDPE, called high density polyethylene, with long straight chains as long as 20, 000 carbons (shown here). It is hard. The other LDPE has many branching chains and is soft


Low density poly(ethene) LDPE © www. chemsheets. co. uk AS 1096 12 -July-2015

High density poly(ethene) HDPE © www. chemsheets. co. uk AS 1096 12 -July-2015

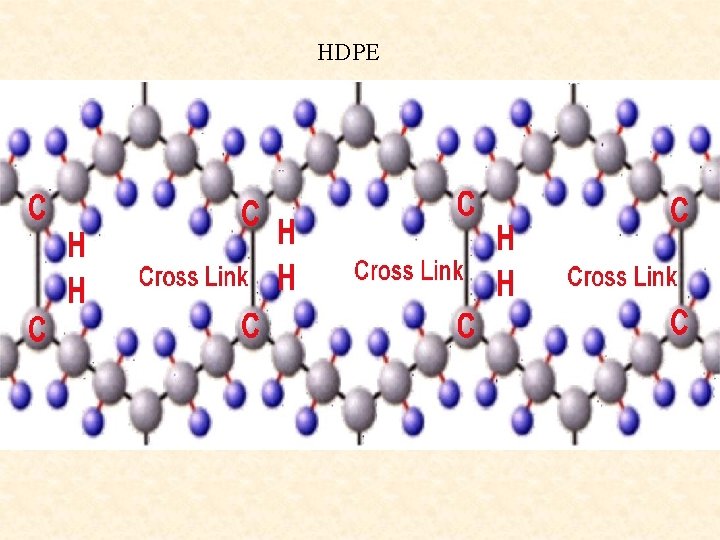
HDPE


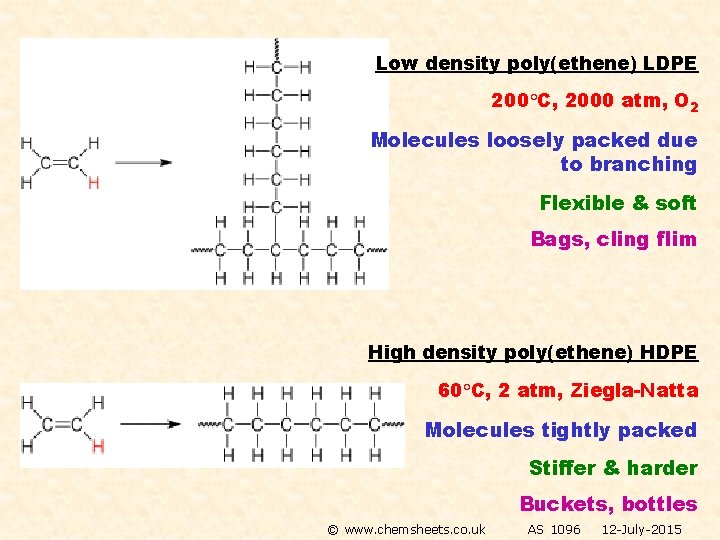
Low density poly(ethene) LDPE 200 C, 2000 atm, O 2 Molecules loosely packed due to branching Flexible & soft Bags, cling flim High density poly(ethene) HDPE 60 C, 2 atm, Ziegla-Natta Molecules tightly packed Stiffer & harder Buckets, bottles © www. chemsheets. co. uk AS 1096 12 -July-2015

Polymerisation of Ethene https: //www. youtube. com/watch? v=sk 6 h 4 oa. Ar. E 0

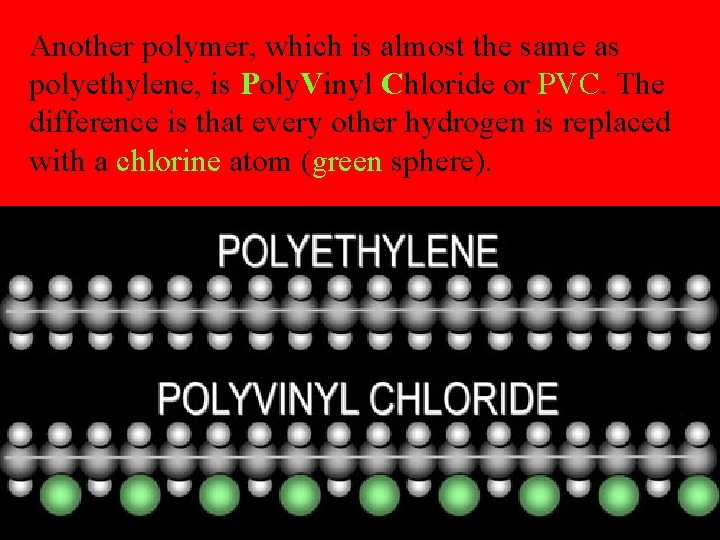
Another polymer, which is almost the same as polyethylene, is Poly. Vinyl Chloride or PVC. The difference is that every other hydrogen is replaced with a chlorine atom (green sphere).

© www. chemsheets. co. uk AS 1096 12 -July-2015

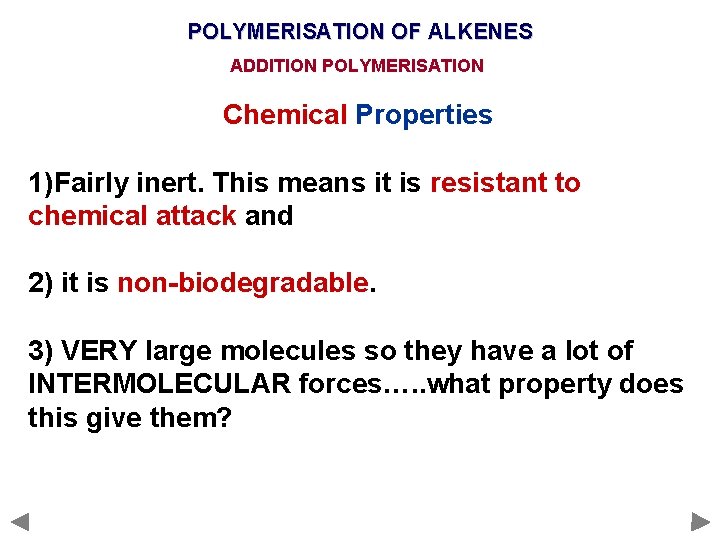
POLYMERISATION OF ALKENES ADDITION POLYMERISATION Chemical Properties 1)Fairly inert. This means it is resistant to chemical attack and 2) it is non-biodegradable. 3) VERY large molecules so they have a lot of INTERMOLECULAR forces…. . what property does this give them?

POLYMER PROPERTIES 1. Plastics are all solid at room temperature, why? These molecules are VERY large so intermolecular forces are huge. so polymers are solid at room temperature. 2. Why do plastics melt rather than react? The carbon–carbon covalent bonds are strong and there are so very many, so plastics are NOT chemically reactive. The physical properties come from weak intermolecular Forces, but here are many of these too, thus plastics melt but ay a fairly high temperatures Ex: 150 0 C (polyethene). Unless they are cross bridged (we talk later)

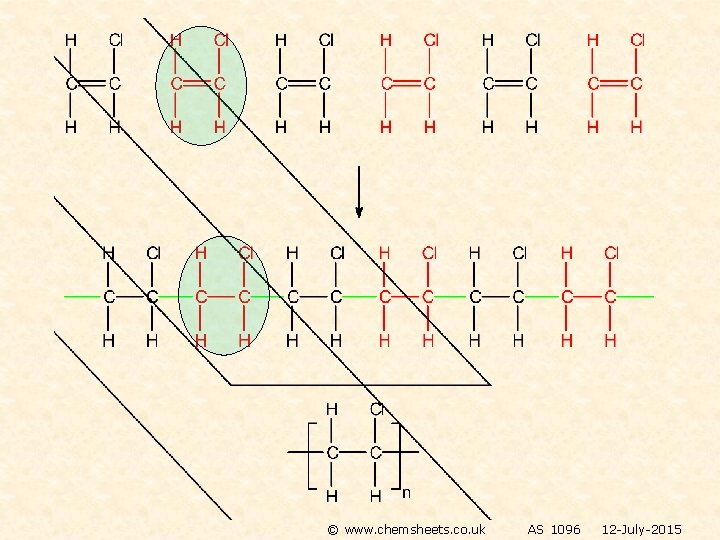
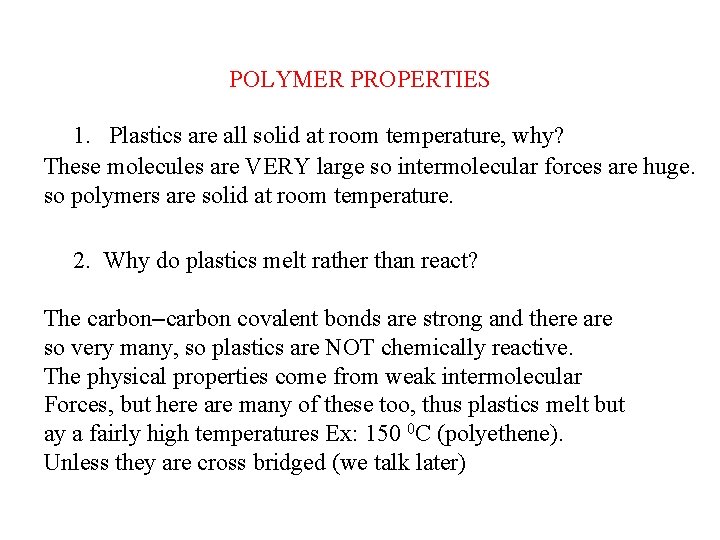
DIFFERENT KINDS OF PLASTIC EXAMPLES OF ADDITION POLYMERISATION ETHENE PROPENE CHLOROETHENE POLY(ETHENE) POLY(PROPENE) POLY(CHLOROETHENE) POLYVINYLCHLORIDE TETRAFLUOROETHENE PVC POLY(TETRAFLUOROETHENE) PTFE “Teflon”

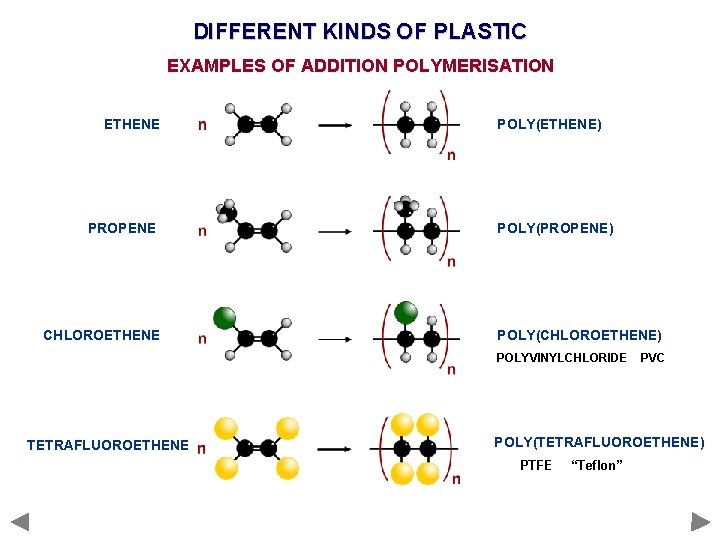
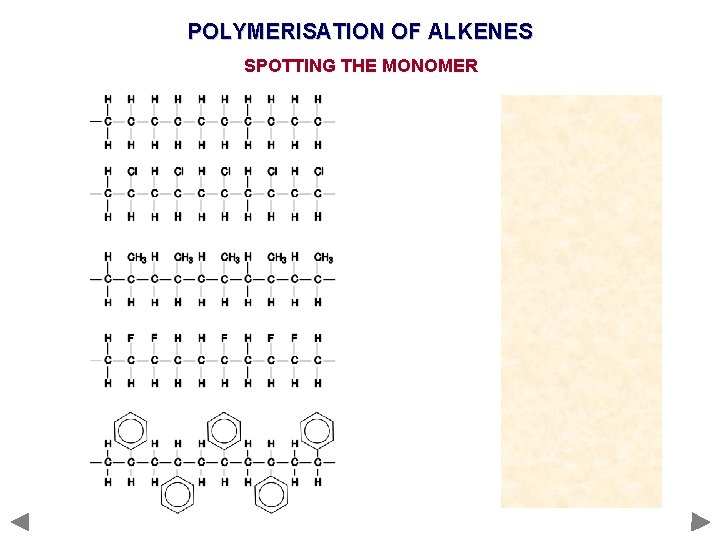
POLYMERISATION OF ALKENES SPOTTING THE MONOMER

POLYMERISATION OF ALKENES SPOTTING THE MONOMER

Polymerisation of Propene https: //www. youtube. com/watch? v=nz 1 uc. I 6 g. CIg

© www. chemsheets. co. uk AS 1096 12 -July-2015

poly(propene) © www. chemsheets. co. uk AS 1096 12 -July-2015

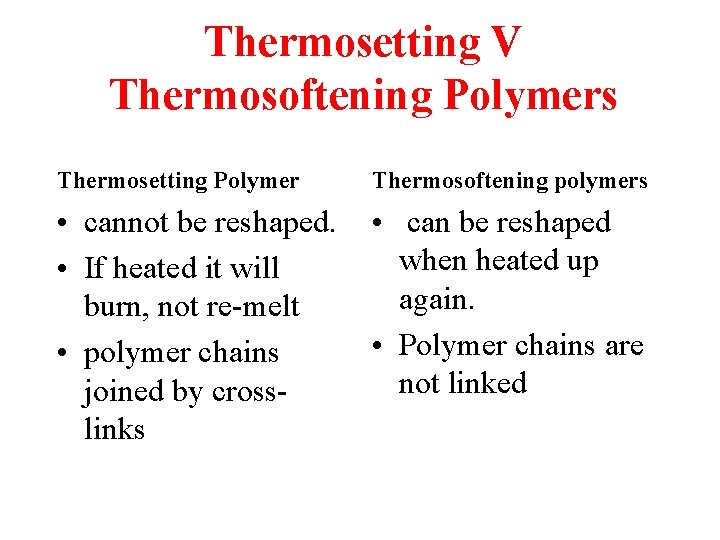
Thermosetting V Thermosoftening Polymers Thermosetting Polymer Thermosoftening polymers • cannot be reshaped. • If heated it will burn, not re-melt • polymer chains joined by crosslinks • can be reshaped when heated up again. • Polymer chains are not linked

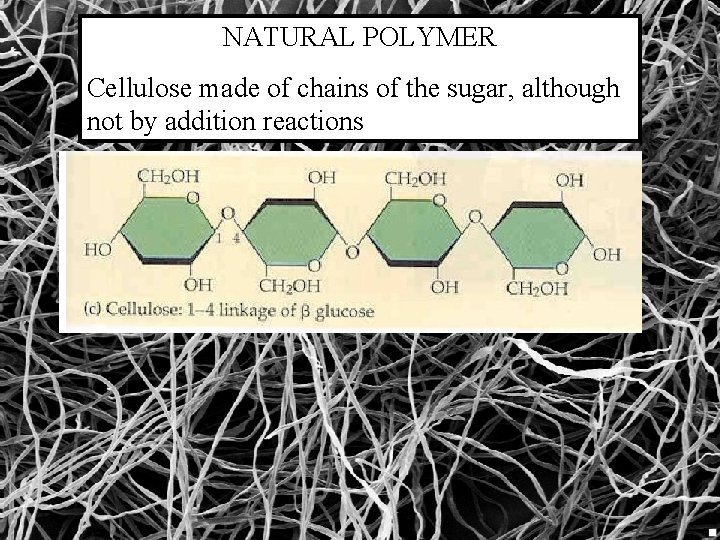
NATURAL POLYMER Cellulose made of chains of the sugar, although not by addition reactions

Plastic can be bad

Plastics Uses and Problems • https: //www. youtube. com/watch? v=e. Zi. BAkb. CR 0 E

Disadvantages of Plastic 1)Most plastics are non-biodegradable. 2) During combustion toxic fumes are released. 3) Plastics are made from fossil fuels which are non-renewable

Burning some plastics is bad (CH 2 CHCl)n + O 2 CO 2 + CO + HCl + H 2 O PVC pipes are safe until it burns. The chlorines in the PVC combine with the hydrogen atoms in the PVC to form hydrogen chloride gas (HCl). When this contacts water in lungs or mouth, it turns to hydrochloric acid (HCl(aq)).

Advantages of Plastics 1)They are cheap and easy to make. 2)They don’t oxidize (rust). 3)Plastics are resistant to chemical attack 4)They are easy to mould into shape and colour 5)They last a lot longer than many metals.

Biodegradable Plastics Instead of using fossil fuel derived monomers for polymerization, Starch from foods (corn starch or potato starch can be used, they can polymerize and form plastics as well as ethene or propene. Advantage: pollution control as these will rot in less than a year Disadvantage: using food when the world still has hungry people is grossly unethical. These plastics do not work as well

Plastics How They are Made https: //www. youtube. com/watch? v=Jarary. OXa 0 Q

Quiz http: //www. bbc. co. uk/education/guides/zxm 39 j 6/activity


STOPPING POLYMERIZATION REACTIONS How can the polymerization reaction end or be stopped? 1)INITIATION, free radical formation via a catalyst benzoyl peroxide 2)PROPAGATION, the reaction keeps going when the ethene forms a free radical upon collision with benzoyl peroxide 3)TERMINATION 3 ways i)Run out or reactants (monomers) ii) 2 free radial monomers combine head to head iii) Addition of chain terminating agent such as nitro benzene