Polymer Synthesis CHEM 421 Ringopening Polymerization II Cyclic
![Why Caprolactam? [H] [O] Polymer Synthesis CHEM 421 NH 2 OH oxime hydroxyl amine Why Caprolactam? [H] [O] Polymer Synthesis CHEM 421 NH 2 OH oxime hydroxyl amine](https://slidetodoc.com/presentation_image_h/3620724b368b9e85487c839c52869bdb/image-9.jpg)







- Slides: 33

Polymer Synthesis CHEM 421 Ring-opening Polymerization (II)

Cyclic Ethers Polymer Synthesis CHEM 421 polymerization difficult (substituents usually will prevent it) unreactive tertrahydropyran dioxane

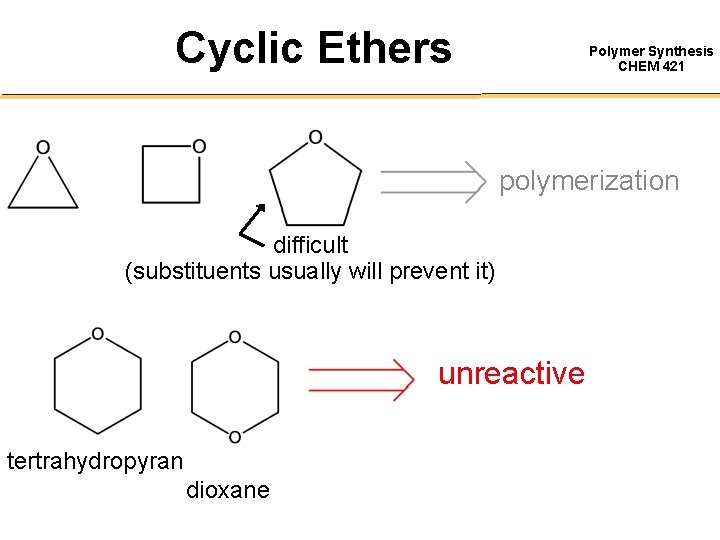
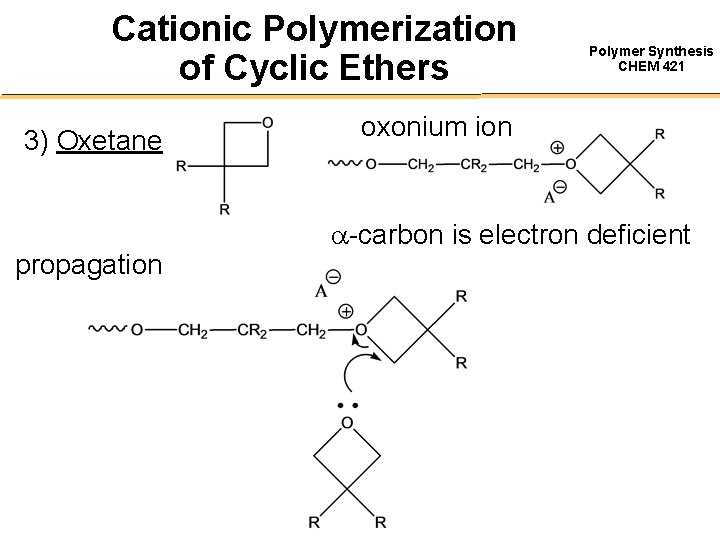
Cationic Polymerization of Cyclic Ethers 3) Oxetane propagation Polymer Synthesis CHEM 421 oxonium ion -carbon is electron deficient

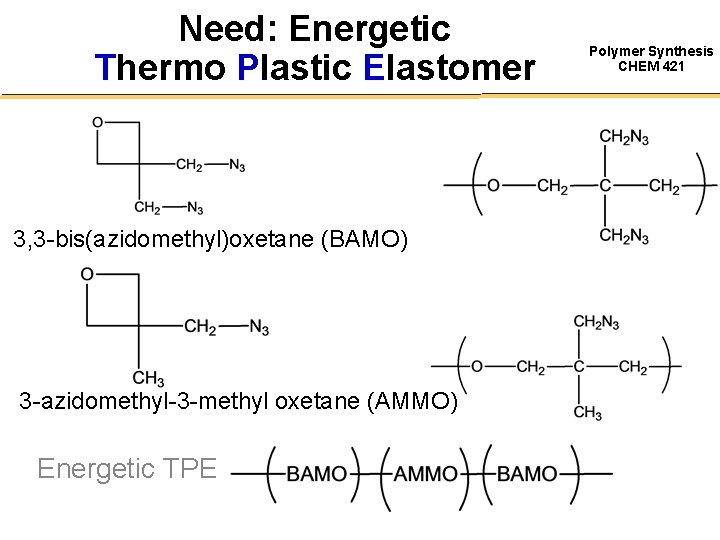
Need: Energetic Thermo Plastic Elastomer 3, 3 -bis(azidomethyl)oxetane (BAMO) 3 -azidomethyl-3 -methyl oxetane (AMMO) Energetic TPE Polymer Synthesis CHEM 421

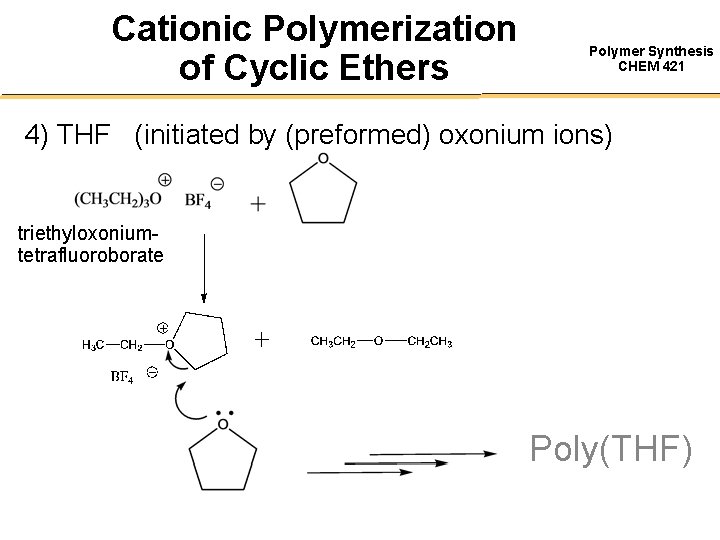
Cationic Polymerization of Cyclic Ethers Polymer Synthesis CHEM 421 4) THF (initiated by (preformed) oxonium ions) triethyloxoniumtetrafluoroborate Poly(THF)

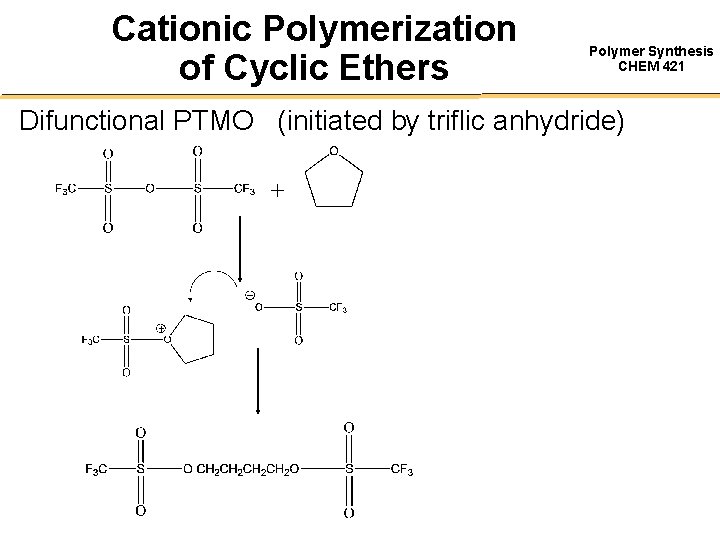
Cationic Polymerization of Cyclic Ethers Polymer Synthesis CHEM 421 Difunctional PTMO (initiated by triflic anhydride)

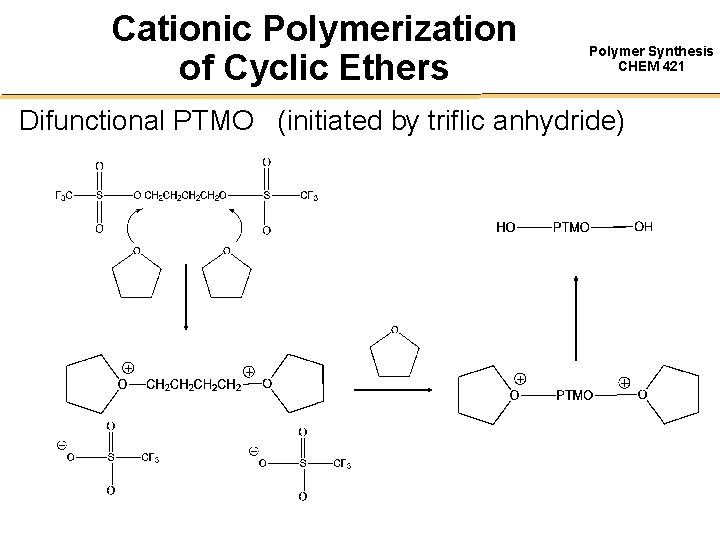
Cationic Polymerization of Cyclic Ethers Polymer Synthesis CHEM 421 Difunctional PTMO (initiated by triflic anhydride)

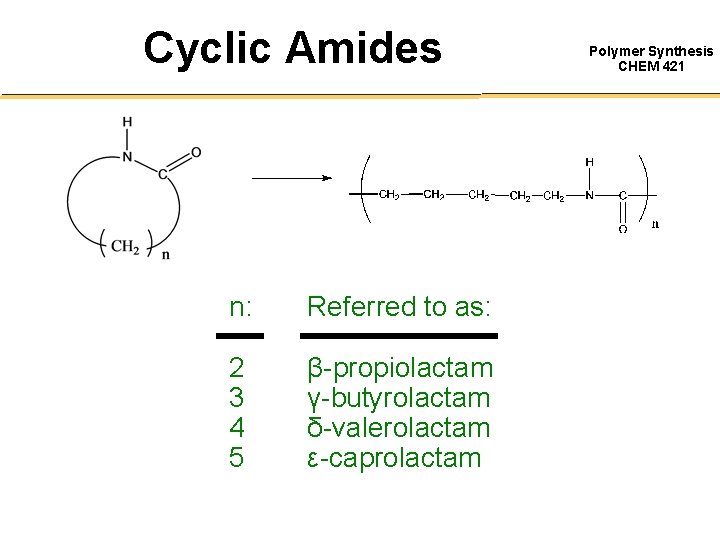
Cyclic Amides n: Referred to as: 2 3 4 5 β-propiolactam γ-butyrolactam δ-valerolactam ε-caprolactam Polymer Synthesis CHEM 421
![Why Caprolactam H O Polymer Synthesis CHEM 421 NH 2 OH oxime hydroxyl amine Why Caprolactam? [H] [O] Polymer Synthesis CHEM 421 NH 2 OH oxime hydroxyl amine](https://slidetodoc.com/presentation_image_h/3620724b368b9e85487c839c52869bdb/image-9.jpg)
Why Caprolactam? [H] [O] Polymer Synthesis CHEM 421 NH 2 OH oxime hydroxyl amine H 2 SO 4 Beckmann Rearrangement H 2 O - H+ tautomerization

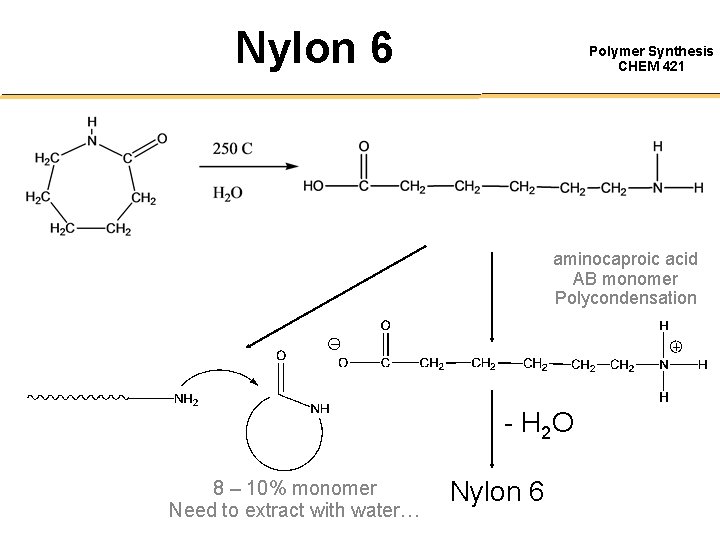
Nylon 6 Polymer Synthesis CHEM 421 aminocaproic acid AB monomer Polycondensation - H 2 O 8 – 10% monomer Need to extract with water… Nylon 6

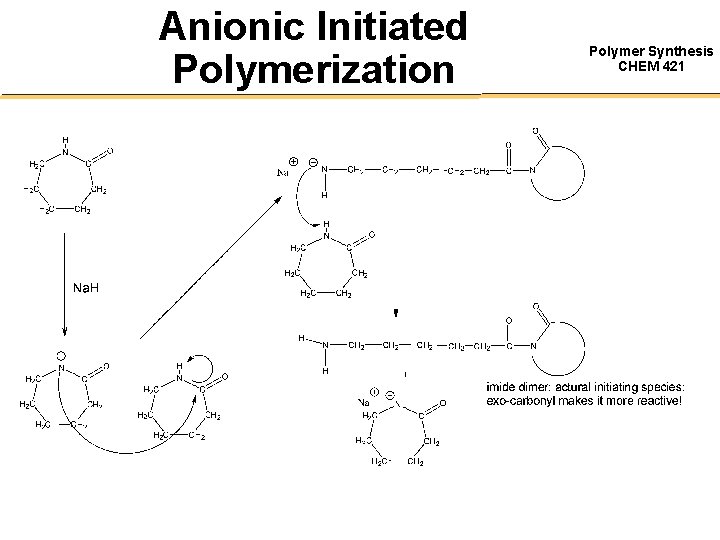
Anionic Initiated Polymerization Polymer Synthesis CHEM 421

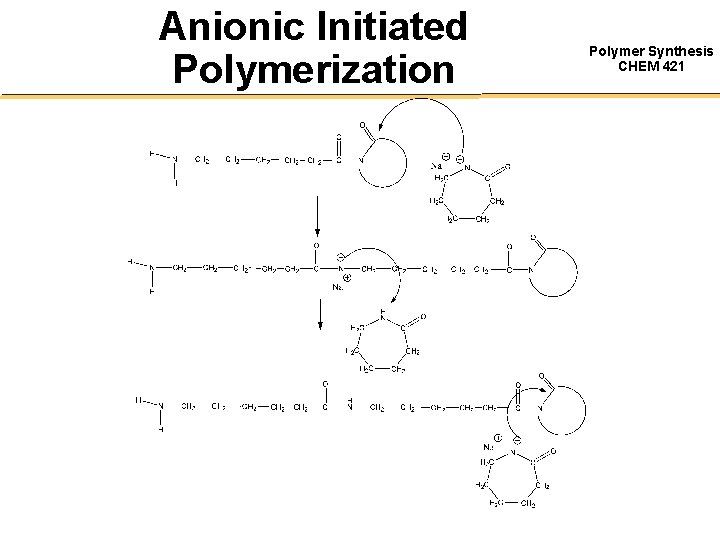
Anionic Initiated Polymerization Polymer Synthesis CHEM 421

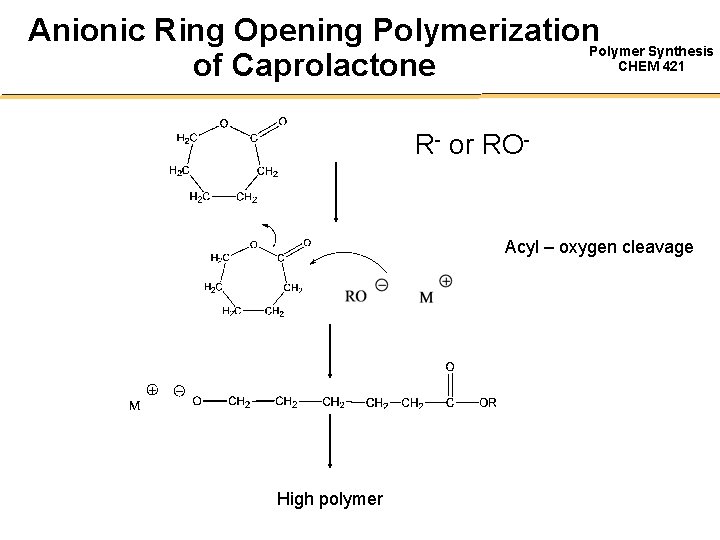
Anionic Ring Opening Polymerization. Polymer Synthesis CHEM 421 of Caprolactone R- or RO- Acyl – oxygen cleavage High polymer

Polylactic Acid (PLA) Polymer Synthesis CHEM 421 • PLA belongs to the family of aliphatic polyester, and it is considered biodegradable and compostable. (i. e. ability of degrading a material under the action of microorganism in a humid environment to produce biomass and carbon dioxide) n PLA is a thermoplastic, high strength, high modulus polymer which can be made from annually renewable resources to yield articles for use in either the industrial packaging field or the biocompatible / bioabsorbable medical device market.

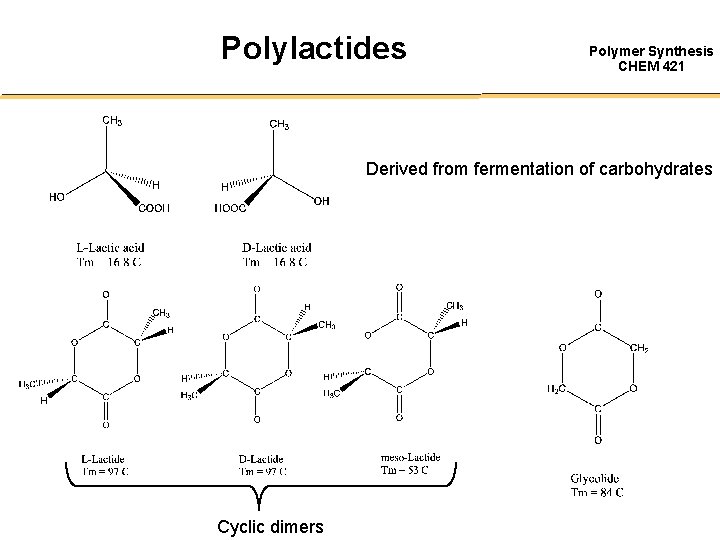
Polylactides Polymer Synthesis CHEM 421 Derived from fermentation of carbohydrates Cyclic dimers

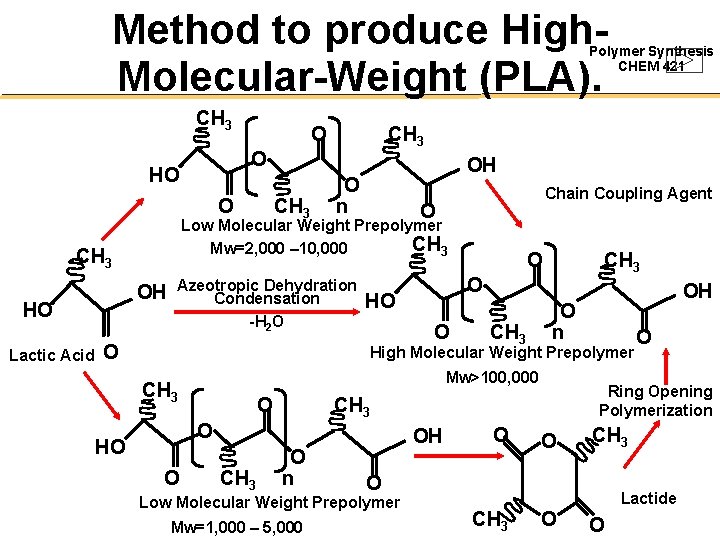
Method to produce High. Molecular-Weight (PLA). Polymer Synthesis CHEM 421 CH 3 O O HO O Lactic Acid CH 3 OH O n Chain Coupling Agent O Low Molecular Weight Prepolymer CH 3 Mw=2, 000 – 10, 000 CH 3 OH HO CH 3 Azeotropic Dehydration Condensation -H 2 O O O CH 3 O HO O CH 3 OH O n High Molecular Weight Prepolymer Mw>100, 000 CH 3 O HO O CH 3 Ring Opening Polymerization CH 3 O O n OH O CH 3 O Low Molecular Weight Prepolymer Mw=1, 000 – 5, 000 O O Lactide CH 3 O O

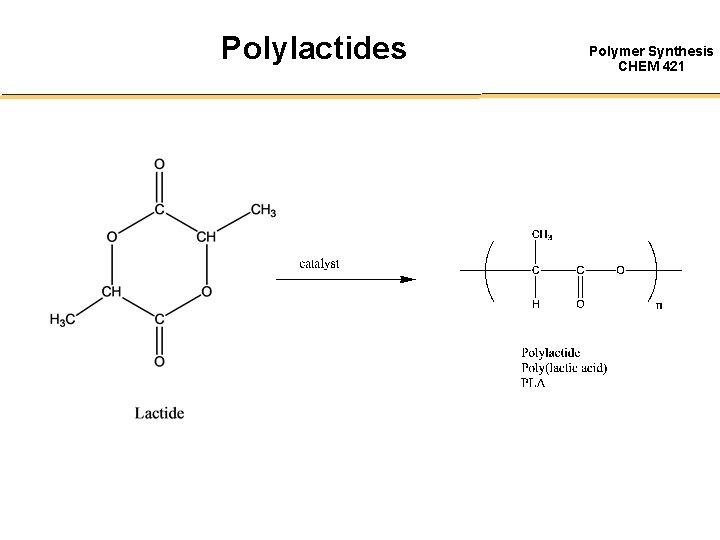
Polylactides Polymer Synthesis CHEM 421

Ring Opening Lactide Polymerization Polymer Synthesis CHEM 421 1) Cationic Polymerization a) Protic Acid (HBr, HCl, triflic acid, etc) b) Lewis acid (Zn. Cl 2 Al. Cl 3, etc) c) Alkilating or Acylating agents (Et 3 O+BF 4, etc) 2) Anionic Polymerization Proceed by nucleophilic reaction of the anion with the carbonyl and the subsequent acyl-oxygen cleavage, this produces an alkoxide end group which continuous propagate. 3) Coordination / Insertion Polymerization Use less reactive metal carboxylates, oxides, and alkoxides. Polymerization by tin, zinc, aluminum, and other heavy metal catalysts with thin (II) and zinc yielding the purest polymers.

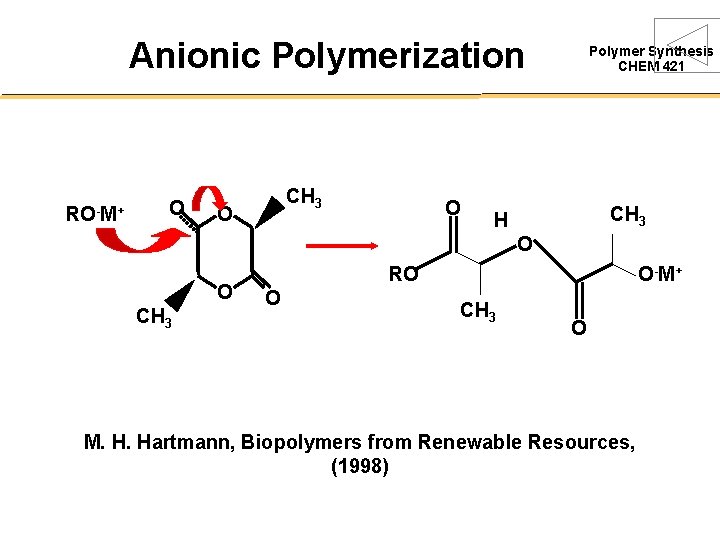
Anionic Polymerization RO-M+ O CH 3 O O Polymer Synthesis CHEM 421 CH 3 H O O CH 3 RO O O-M+ CH 3 O M. H. Hartmann, Biopolymers from Renewable Resources, (1998)

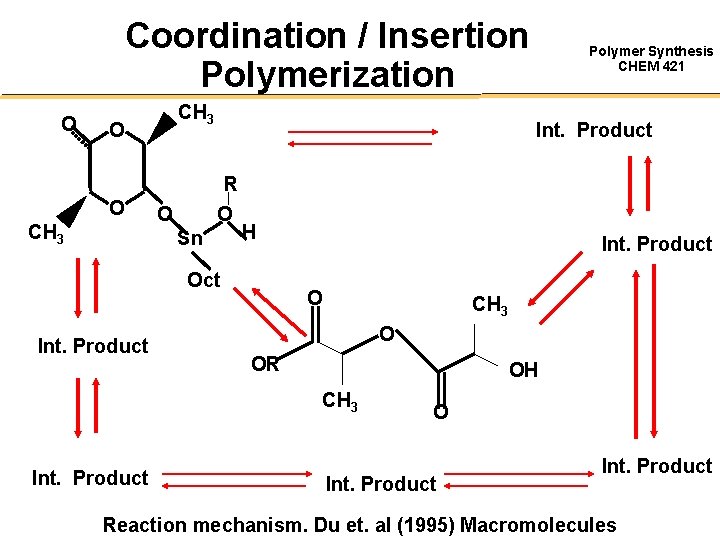
Coordination / Insertion Polymerization O CH 3 O O CH 3 Int. Product R O O Sn H Oct Int. Product O CH 3 O OR OH CH 3 Int. Product Polymer Synthesis CHEM 421 O Int. Product Reaction mechanism. Du et. al (1995) Macromolecules

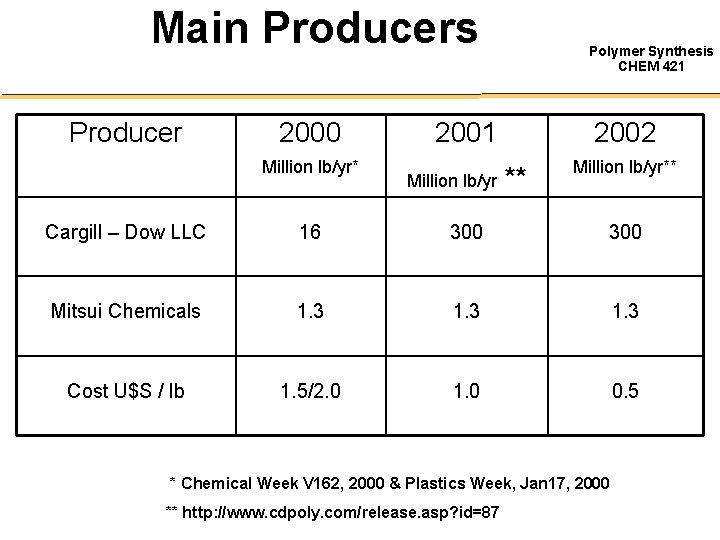
Main Producers Producer 2000 Million lb/yr* Polymer Synthesis CHEM 421 2001 Million lb/yr 2002 ** Million lb/yr** Cargill – Dow LLC 16 300 Mitsui Chemicals 1. 3 Cost U$S / lb 1. 5/2. 0 1. 0 0. 5 * Chemical Week V 162, 2000 & Plastics Week, Jan 17, 2000 ** http: //www. cdpoly. com/release. asp? id=87

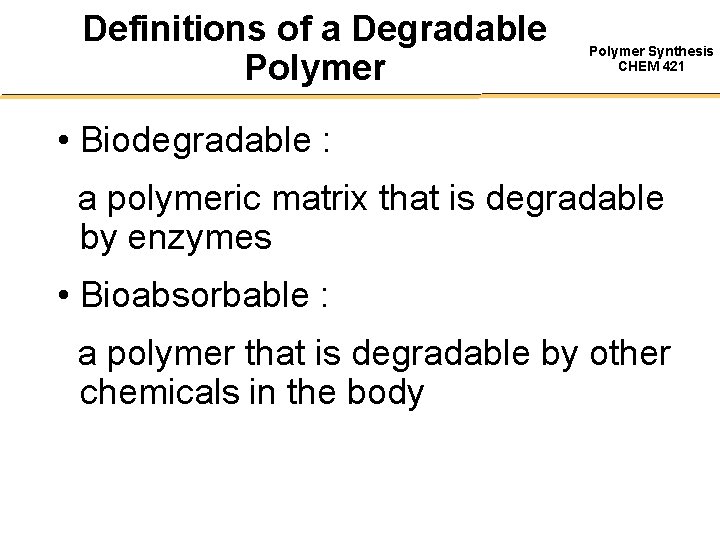
Definitions of a Degradable Polymer Synthesis CHEM 421 • Biodegradable : a polymeric matrix that is degradable by enzymes • Bioabsorbable : a polymer that is degradable by other chemicals in the body

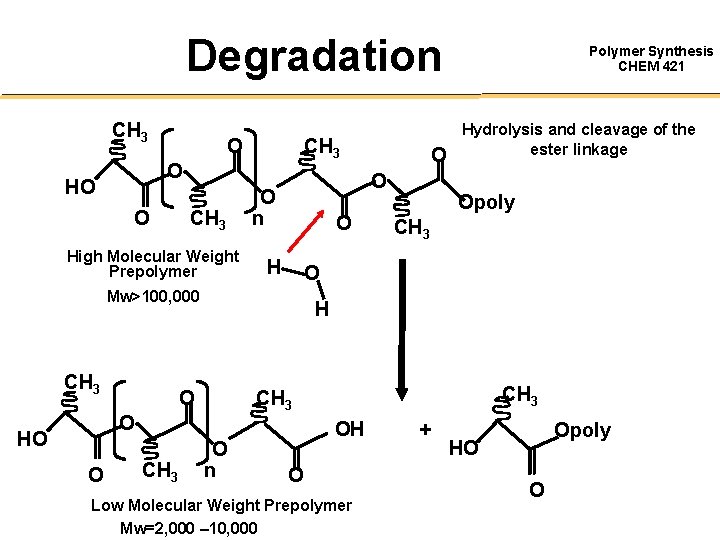
Degradation CH 3 O HO O CH 3 High Molecular Weight Prepolymer O O n O H O O CH 3 Opoly CH 3 H CH 3 O HO Hydrolysis and cleavage of the ester linkage O Mw>100, 000 CH 3 O Polymer Synthesis CHEM 421 O n OH O Low Molecular Weight Prepolymer Mw=2, 000 – 10, 000 + Opoly HO O

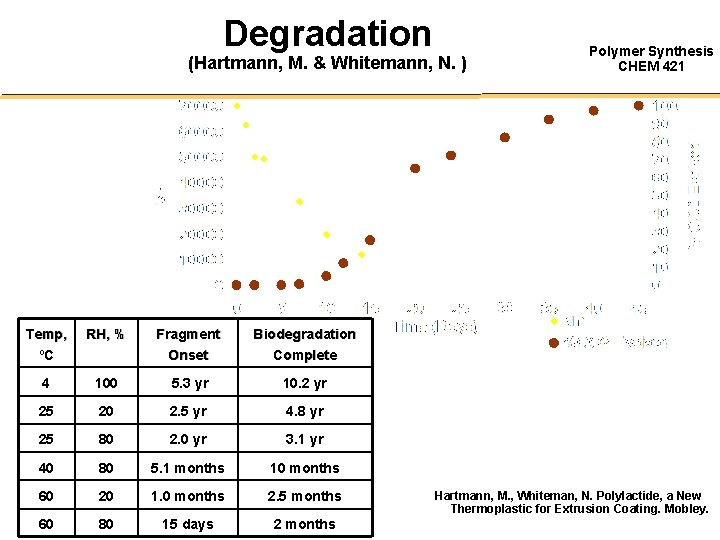
Degradation (Hartmann, M. & Whitemann, N. ) Temp, ºC RH, % Fragment Onset Biodegradation Complete 4 100 5. 3 yr 10. 2 yr 25 20 2. 5 yr 4. 8 yr 25 80 2. 0 yr 3. 1 yr 40 80 5. 1 months 10 months 60 20 1. 0 months 2. 5 months 60 80 15 days 2 months Polymer Synthesis CHEM 421 Hartmann, M. , Whiteman, N. Polylactide, a New Thermoplastic for Extrusion Coating. Mobley.

Factors Which Affect Polymer Degradation Polymer Synthesis CHEM 421 • Chemical composition. • Distribution of repeated units. • Presence of unexpected units or chain defects. • Stereochemistry. • Molecular weight distribution. • Morphology (amorphous/semicrystalline).

The “Ideal” Polymer for Degradable Implants and Drug Delivery Polymer Synthesis CHEM 421 • A degradable polymer: the polymer matrix should degrade into smaller fragments over a period of time, which then can be excreted from the body • The degradation products of the polymer should not contain or become toxic materials for the body • (An ideal mechanism for release of the drug should be at a constant rate )

Factors that Accelerate Polymer Degradation Polymer Synthesis CHEM 421 • More hydrophilic backbones and/or endgroups. • More reactive hydrolytic groups in the backbone. • Less crystallinity. • More porosity. • Smaller size device.

Polymeric drug delivery Polymer Synthesis CHEM 421 • Drugs are embedded in a polymeric matrix so that they may be protected from reactions by enzymes and body chemical substances that can destroy the drug chemistry before it reaches the targeted areas. • The polymeric matrix also functions as a time controller in the concentration of active ingredient release.

Polylactides • Biodegradable Polymer Wafer • Roughly the size of a dime, biodegradable polymer wafers (right) can be implanted in specific places in the brain after cancer surgery to deliver cancer-killing drugs at a controlled rate. (Photo courtesy of Guilford Pharmaceuticals) Polymer Synthesis CHEM 421

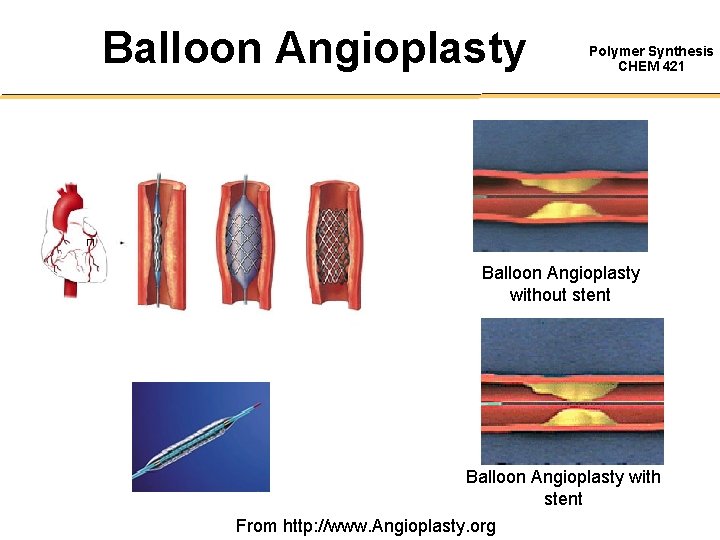
Balloon Angioplasty Polymer Synthesis CHEM 421 Balloon Angioplasty without stent Balloon Angioplasty with stent From http: //www. Angioplasty. org

Problems With Stents • Restenosis - A condition in which a stented artery becomes clogged with scar tissue that has grown through the mesh in a stent. • Current Treatment Occasionally doctors can perform a second balloon angioplasty, but there is a risk of the second balloon getting snagged on the old stent. The most common treatment for restenosis is to insert a drilllike instrument into the clogged artery, which is much more invasive than angioplasty. Rotoblation Cutting Balloon Pictures from www. clevelandclinic. org Polymer Synthesis CHEM 421

2 nd Generation Stents: Drug delivery Coating on Metal Stents Polymer Synthesis CHEM 421 • STENT DESIGN: Biosensors’ highly successful S-Stent/RX 1 delivery platform featuring uniform strut geometry for optimized drug distribution, to which has been added an ultra-thin, proprietary resorbable polymer coating containing the drug. • DRUG TYPE: EVEROLIMUS: A Rapamycin analogue previously studied for organ transplant applications, possessing potent smooth muscle cell immunosuppressive and anti-proliferative properties. • INHIBITION OF RESTENOSIS: The drug eluting stent eliminates restenosis compared to bare metal control stents implanted in pigs using the coronary overstretch injury model. http: //www. biosensors. com. sg/challenge/Main. Page. html

3 rd Generation Stents: Totally Polymer Synthesis Bioabsorbable, Drug-Eluting Stents CHEM 421 • Make the stent entirely from a bioabsorbable material – No “chest full of metal…” – Structural support only needed for “months” – No prolonged blocking of vessel branches – Higher drug loading possible – MRI delivery/monitoring possible – Other applications