Heterogeneous Polymerizations Precipitation Suspension Dispersion Emulsion Polymer Synthesis
![GPC Traces - Effect of [VF 2] on MWD Polymer Synthesis CHEM 421 75 GPC Traces - Effect of [VF 2] on MWD Polymer Synthesis CHEM 421 75](https://slidetodoc.com/presentation_image/5858e468c72458b7923a72225f458b67/image-7.jpg)


- Slides: 23

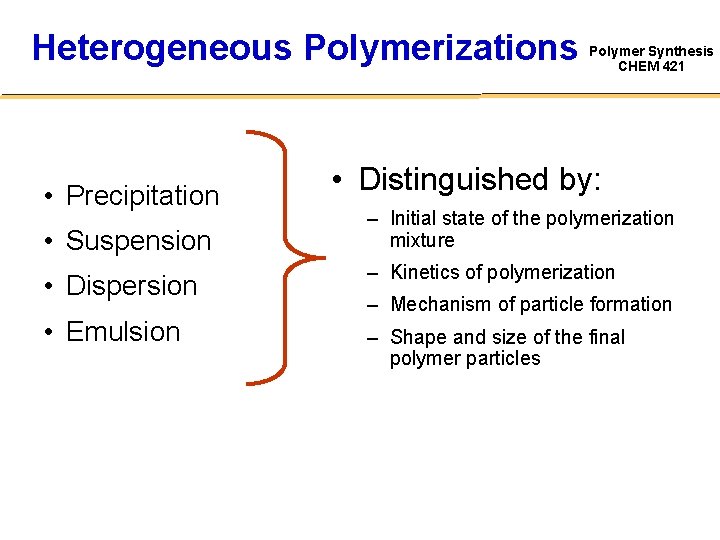
Heterogeneous Polymerizations • Precipitation • Suspension • Dispersion • Emulsion Polymer Synthesis CHEM 421 • Distinguished by: – Initial state of the polymerization mixture – Kinetics of polymerization – Mechanism of particle formation – Shape and size of the final polymer particles

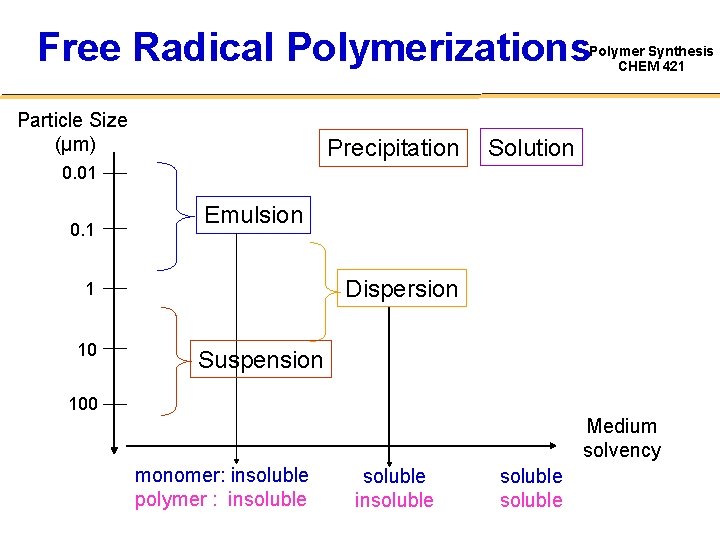
Free Radical Polymerizations Polymer Synthesis CHEM 421 Particle Size (µm) Precipitation Solution 0. 01 0. 1 Emulsion Dispersion 1 10 Suspension 100 Medium solvency monomer: insoluble polymer : insoluble soluble

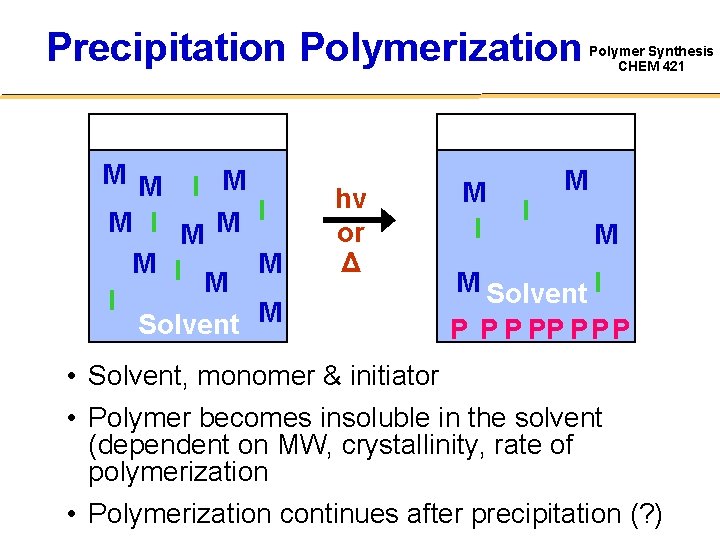
Precipitation Polymerization M M I M M I Solvent M hν or Δ M I I Polymer Synthesis CHEM 421 M M M Solvent I P PP P • Solvent, monomer & initiator • Polymer becomes insoluble in the solvent (dependent on MW, crystallinity, rate of polymerization • Polymerization continues after precipitation (? )

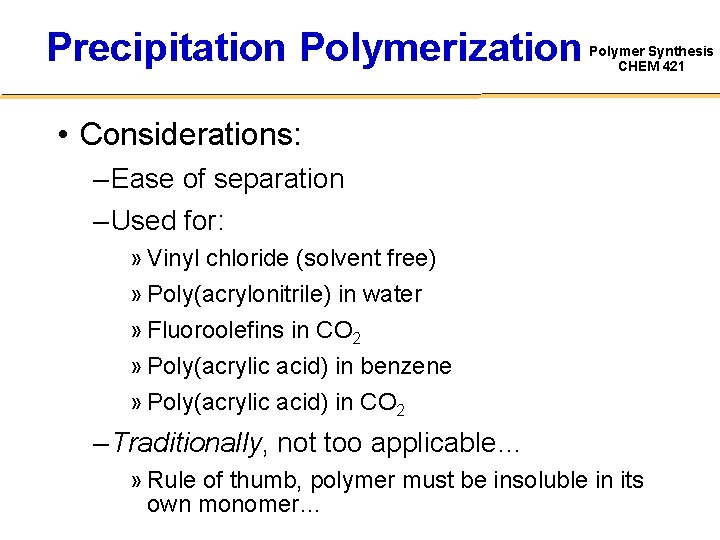
Precipitation Polymerization Polymer Synthesis CHEM 421 • Considerations: – Ease of separation – Used for: » Vinyl chloride (solvent free) » Poly(acrylonitrile) in water » Fluoroolefins in CO 2 » Poly(acrylic acid) in benzene » Poly(acrylic acid) in CO 2 – Traditionally, not too applicable… » Rule of thumb, polymer must be insoluble in its own monomer…

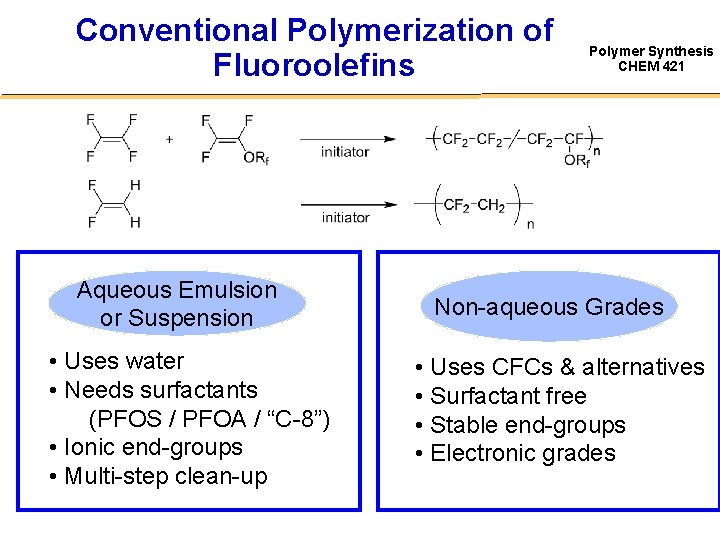
Conventional Polymerization of Fluoroolefins Aqueous Emulsion or Suspension • Uses water • Needs surfactants (PFOS / PFOA / “C-8”) • Ionic end-groups • Multi-step clean-up Polymer Synthesis CHEM 421 Non-aqueous Grades • Uses CFCs & alternatives • Surfactant free • Stable end-groups • Electronic grades

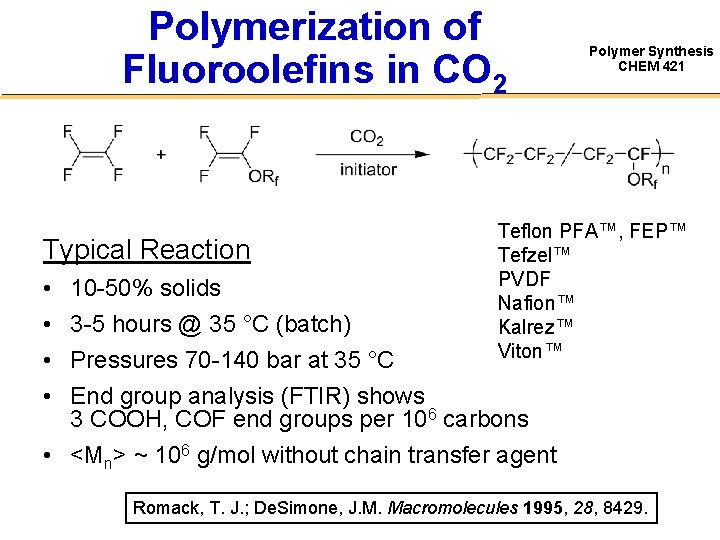
Polymerization of Fluoroolefins in CO 2 Typical Reaction • • 10 -50% solids 3 -5 hours @ 35 °C (batch) Polymer Synthesis CHEM 421 Teflon PFA™, FEP™ Tefzel™ PVDF Nafion™ Kalrez™ Viton™ Pressures 70 -140 bar at 35 °C End group analysis (FTIR) shows 3 COOH, COF end groups per 106 carbons • <Mn> ~ 106 g/mol without chain transfer agent Romack, T. J. ; De. Simone, J. M. Macromolecules 1995, 28, 8429.
![GPC Traces Effect of VF 2 on MWD Polymer Synthesis CHEM 421 75 GPC Traces - Effect of [VF 2] on MWD Polymer Synthesis CHEM 421 75](https://slidetodoc.com/presentation_image/5858e468c72458b7923a72225f458b67/image-7.jpg)
GPC Traces - Effect of [VF 2] on MWD Polymer Synthesis CHEM 421 75 °C, 4000 psig, = 20 minutes Bimodal MWDs observed when [VF 2]0 greater than about 1. 9 M

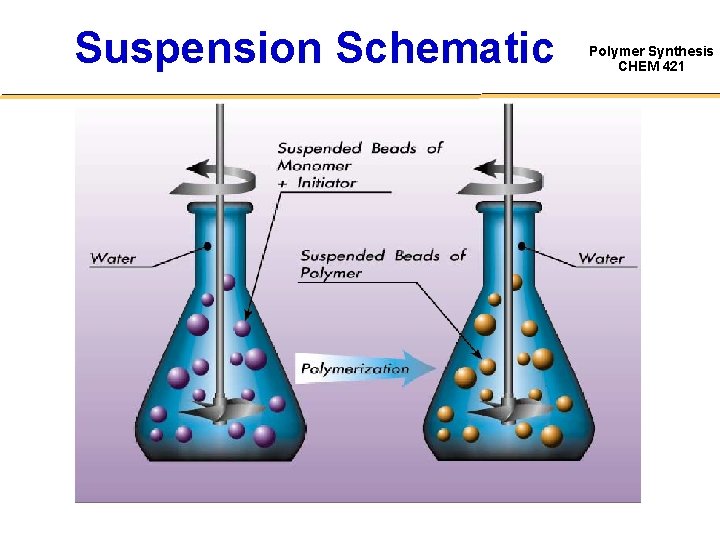
Suspension Schematic Polymer Synthesis CHEM 421

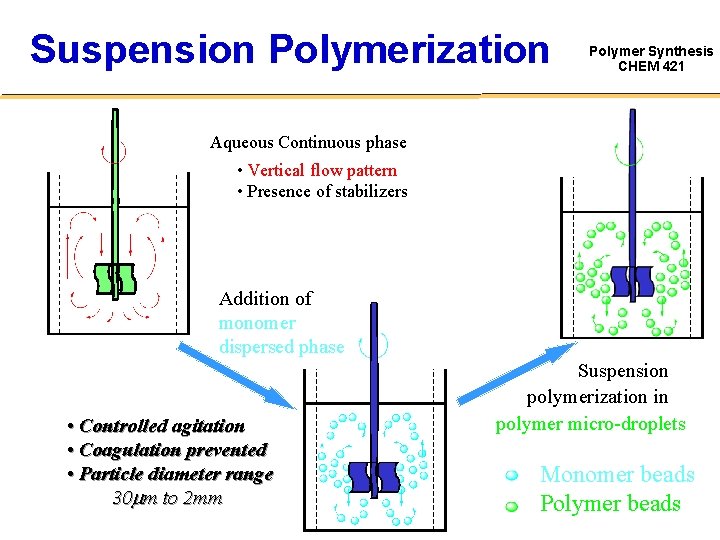
Suspension Polymerization Polymer Synthesis CHEM 421 Aqueous Continuous phase • Vertical flow pattern • Presence of stabilizers Addition of monomer dispersed phase • Controlled agitation • Coagulation prevented • Particle diameter range 30 mm to 2 mm Suspension polymerization in polymer micro-droplets Monomer beads Polymer beads

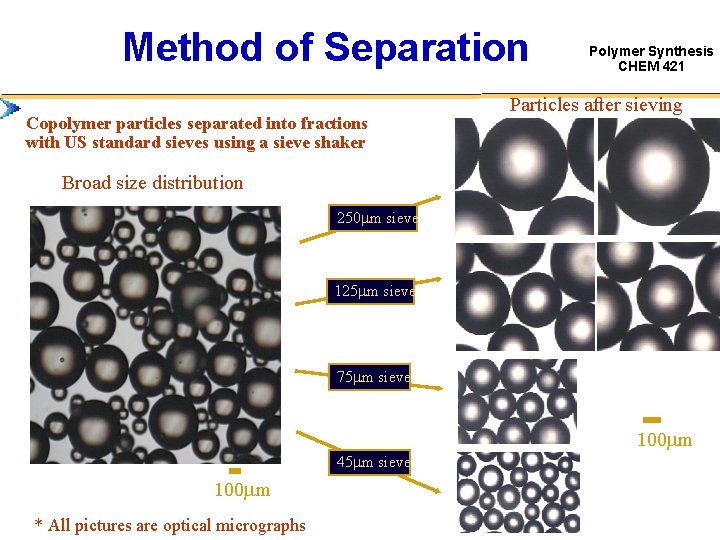
Method of Separation Copolymer particles separated into fractions with US standard sieves using a sieve shaker Polymer Synthesis CHEM 421 Particles after sieving Broad size distribution 250 mm sieve 125 mm sieve 75 mm sieve 100 mm 45 mm sieve 100 mm * All pictures are optical micrographs

Suspension Polymerization Polymer Synthesis CHEM 421 • Considerations: – Stabilizers used: » water-soluble polymers: i. e. poly(vinyl alcohol) – Hard to control particle size – separate with sieves – Two phase system only with shear, can’t recover colloidal system – Used for: styrene, (meth)acrylic esters, vinyl chloride, vinyl acetate » Chromatographic separation media, affinity columns, etc

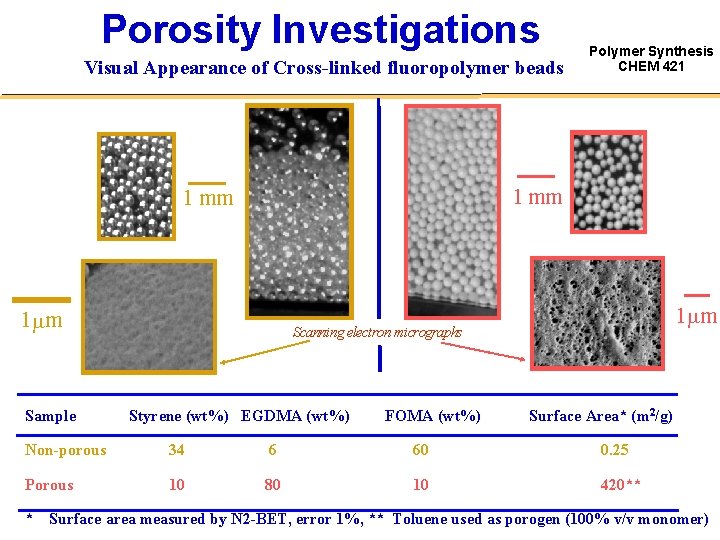
Porosity Investigations Polymer Synthesis CHEM 421 • Application to transition-metal catalysis and enzymatic catalysis Highly porous particles (high specific surface area) will permit an improved activity of the system by increasing the density of actives sites per unit of volume • Porosity potential by incorporating various porogens (solvent, nonsolvent or linear polymer) Toluene has been successfully investigated • Porosity evaluation by performing SEM and N 2 -BET

Porosity Investigations Visual Appearance of Cross-linked fluoropolymer beads 1 mm Sample Polymer Synthesis CHEM 421 1 mm m Scanning electron micrographs Styrene (wt%) EGDMA (wt%) FOMA (wt%) Surface Area* (m 2/g) Non-porous 34 6 60 0. 25 Porous 10 80 10 420** * Surface area measured by N 2 -BET, error 1%, ** Toluene used as porogen (100% v/v monomer)

Potential Utility of CO 2 Polymer Synthesis CHEM 421 • CO 2 is non-toxic, cheap and readily available • CO 2 is a by-product from production of ammonia, ethanol, hydrogen • CO 2 is found in natural reservoirs and used in EOR • Easily of separated and recycled • CO 2 has a low surface tension, low viscosity • Liquid and supercritical states “convenient” • Inert for many chemistries

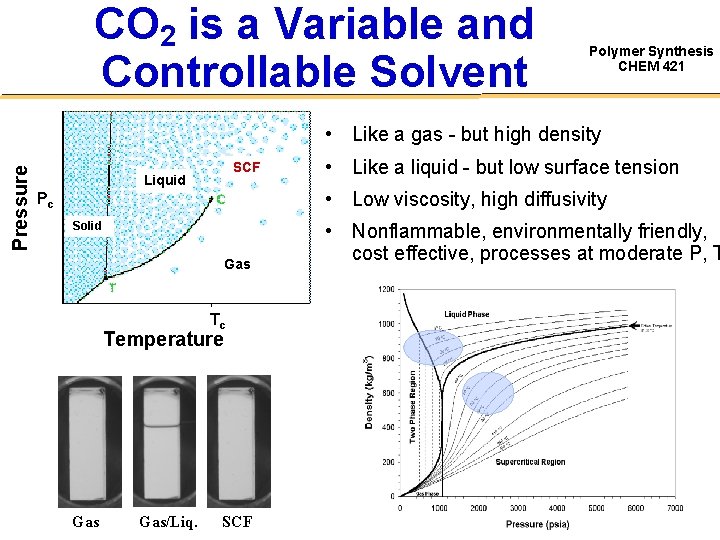
CO 2 is a Variable and Controllable Solvent Polymer Synthesis CHEM 421 Pressure • Like a gas - but high density SCF Liquid • Low viscosity, high diffusivity Pc Solid Gas Tc Temperature Gas/Liq. • Like a liquid - but low surface tension SCF • Nonflammable, environmentally friendly, cost effective, processes at moderate P, T

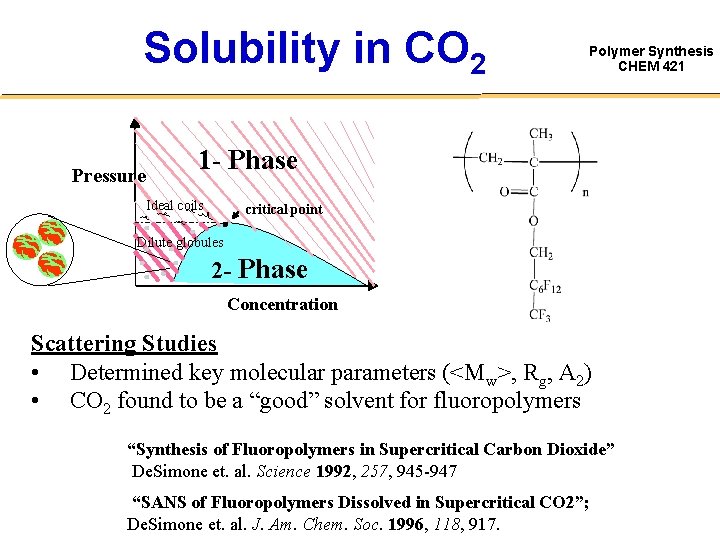
Solubility in CO 2 Pressure Polymer Synthesis CHEM 421 1 - Phase Ideal coils critical point Dilute globules 2 - Phase Concentration Scattering Studies • Determined key molecular parameters (<Mw>, Rg, A 2) • CO 2 found to be a “good” solvent for fluoropolymers “Synthesis of Fluoropolymers in Supercritical Carbon Dioxide” De. Simone et. al. Science 1992, 257, 945 -947 “SANS of Fluoropolymers Dissolved in Supercritical CO 2”; De. Simone et. al. J. Am. Chem. Soc. 1996, 118, 917.

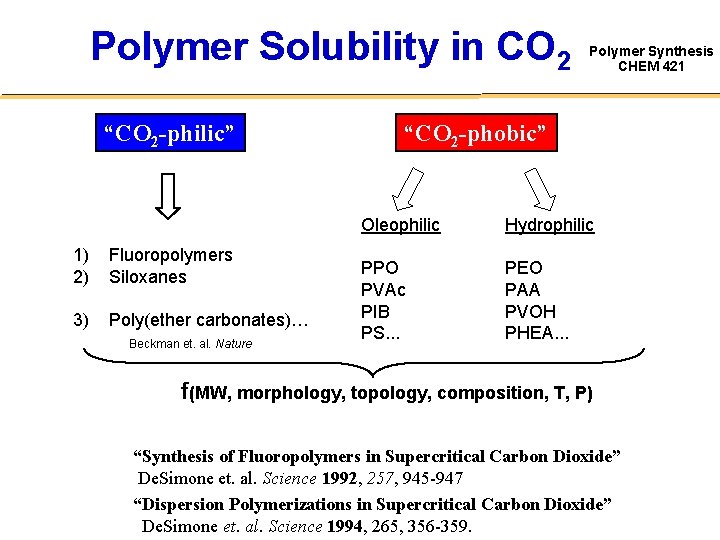
Polymer Solubility in CO 2 “CO 2 -philic” 1) 2) Fluoropolymers Siloxanes 3) Poly(ether carbonates)… Beckman et. al. Nature Polymer Synthesis CHEM 421 “CO 2 -phobic” Oleophilic Hydrophilic PPO PVAc PIB PS. . . PEO PAA PVOH PHEA. . . f(MW, morphology, topology, composition, T, P) “Synthesis of Fluoropolymers in Supercritical Carbon Dioxide” De. Simone et. al. Science 1992, 257, 945 -947 “Dispersion Polymerizations in Supercritical Carbon Dioxide” De. Simone et. al. Science 1994, 265, 356 -359.

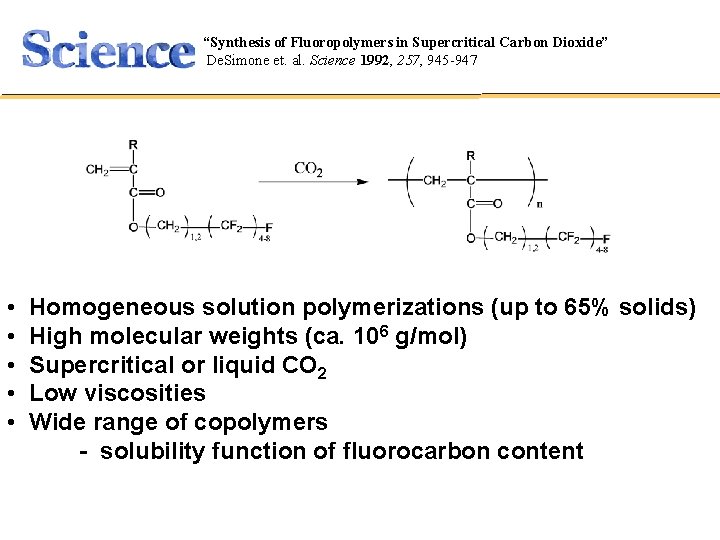
“Synthesis of Fluoropolymers in Supercritical Carbon Dioxide” Polymer Synthesis De. Simone et. al. Science 1992, 257, 945 -947 CHEM 421 • • • Homogeneous solution polymerizations (up to 65% solids) High molecular weights (ca. 106 g/mol) Supercritical or liquid CO 2 Low viscosities Wide range of copolymers - solubility function of fluorocarbon content

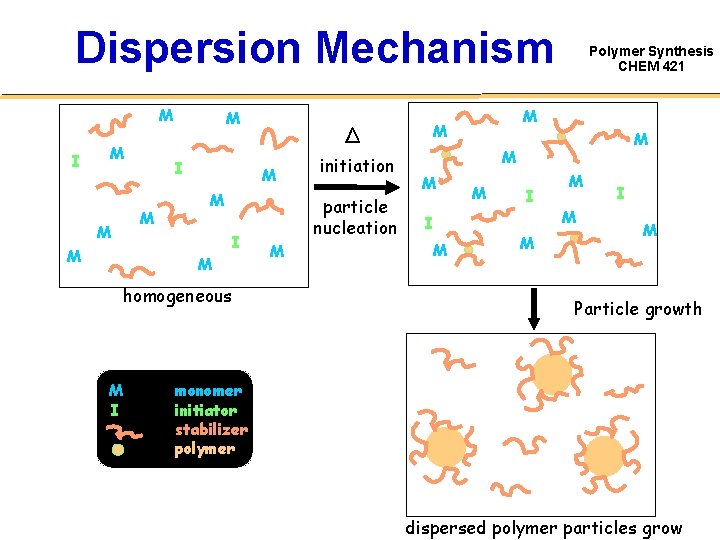
Dispersion Mechanism M I M M M M I homogeneous M I M initiation particle nucleation M M Δ Polymer Synthesis CHEM 421 M M M I M Particle growth monomer initiator stabilizer polymer dispersed polymer particles grow

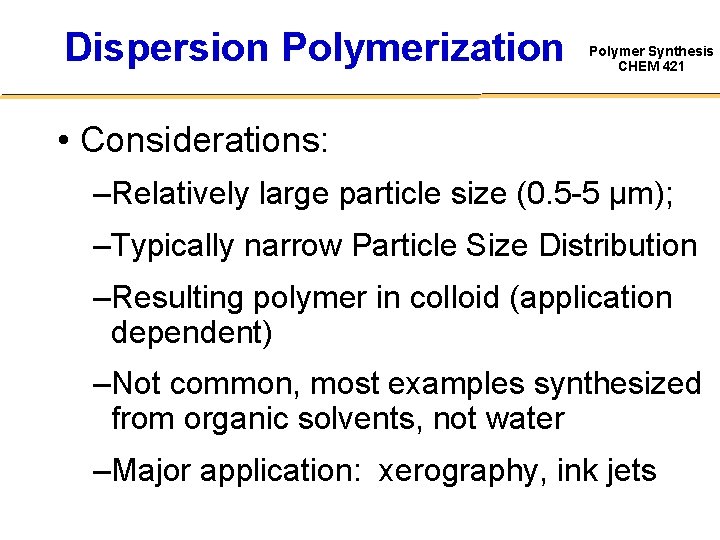
Dispersion Polymerization Polymer Synthesis CHEM 421 • Considerations: –Relatively large particle size (0. 5 -5 μm); –Typically narrow Particle Size Distribution –Resulting polymer in colloid (application dependent) –Not common, most examples synthesized from organic solvents, not water –Major application: xerography, ink jets

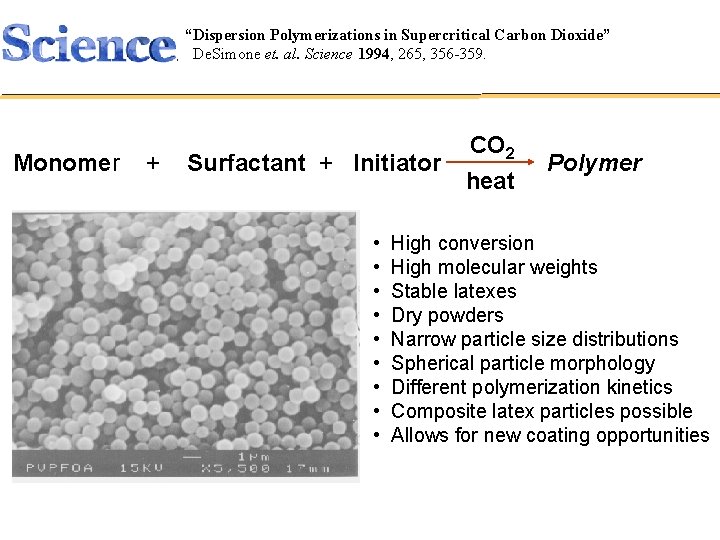
“Dispersion Polymerizations in Supercritical Carbon Dioxide” Polymer Synthesis De. Simone et. al. Science 1994, 265, 356 -359. CHEM 421 Monomer + Surfactant + Initiator • • • CO 2 heat Polymer High conversion High molecular weights Stable latexes Dry powders Narrow particle size distributions Spherical particle morphology Different polymerization kinetics Composite latex particles possible Allows for new coating opportunities

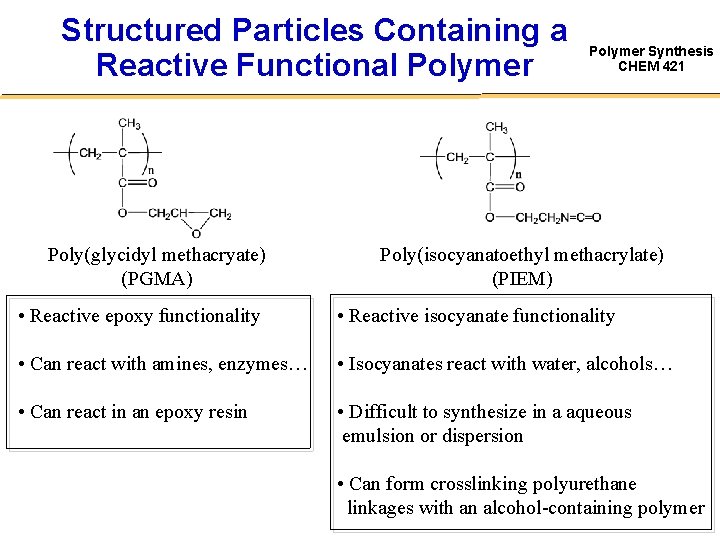
Structured Particles Containing a Reactive Functional Polymer Poly(glycidyl methacryate) (PGMA) Polymer Synthesis CHEM 421 Poly(isocyanatoethyl methacrylate) (PIEM) • Reactive epoxy functionality • Reactive isocyanate functionality • Can react with amines, enzymes… • Isocyanates react with water, alcohols… • Can react in an epoxy resin • Difficult to synthesize in a aqueous emulsion or dispersion • Can form crosslinking polyurethane linkages with an alcohol-containing polymer

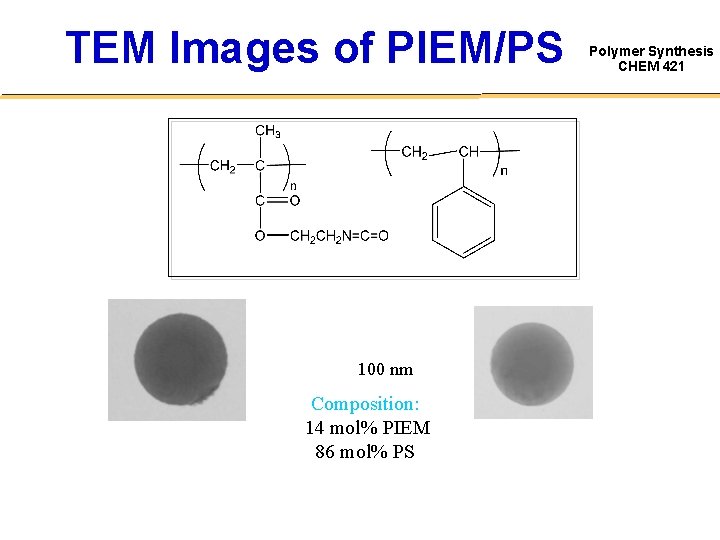
TEM Images of PIEM/PS 100 nm Composition: 14 mol% PIEM 86 mol% PS Polymer Synthesis CHEM 421
 Suspension polymerization
Suspension polymerization Dispersion phase and dispersion medium
Dispersion phase and dispersion medium Pulse dispersion in optical fiber
Pulse dispersion in optical fiber Dispersed phase and dispersion medium
Dispersed phase and dispersion medium Co precipitation and post precipitation
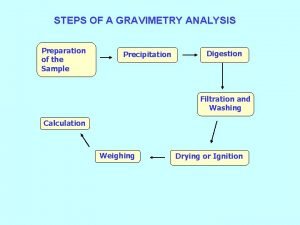
Co precipitation and post precipitation Gravimetry steps
Gravimetry steps Suspension heterogeneous mixture
Suspension heterogeneous mixture Peek polymer synthesis
Peek polymer synthesis Primary emulsion formula
Primary emulsion formula Three ring sling
Three ring sling Heterogeneous transfer learning
Heterogeneous transfer learning What is a heterogeneous mixture that never settles
What is a heterogeneous mixture that never settles Heterogeneous hypoechoic lesion in liver
Heterogeneous hypoechoic lesion in liver Mixture
Mixture Grade 7 science unit 3: mixtures and solutions answers
Grade 7 science unit 3: mixtures and solutions answers Heterogeneous catalyst
Heterogeneous catalyst Homogeneous reaction definition
Homogeneous reaction definition Decanting
Decanting Is supreme pizza homogeneous or heterogeneous
Is supreme pizza homogeneous or heterogeneous Is jello homogeneous or heterogeneous
Is jello homogeneous or heterogeneous Heterogenous team
Heterogenous team Maple syrup homogeneous or heterogeneous
Maple syrup homogeneous or heterogeneous Is tap water a heterogeneous mixture
Is tap water a heterogeneous mixture Homogeneous mixture
Homogeneous mixture