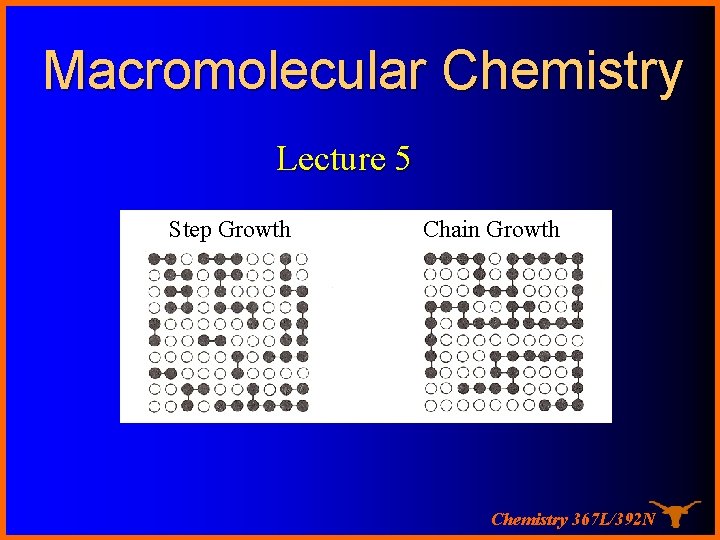
Macromolecular Chemistry Lecture 5 Step Growth Chain Growth




- Slides: 26

Macromolecular Chemistry Lecture 5 Step Growth Chain Growth Chemistry 367 L/392 N

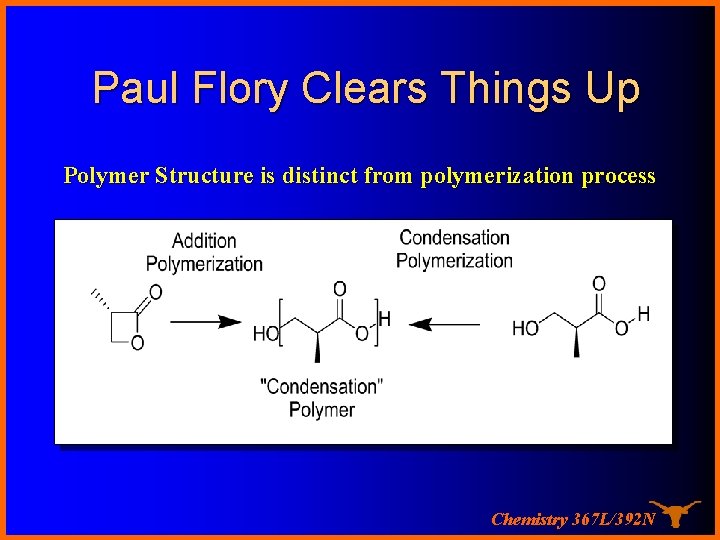
Paul Flory Clears Things Up Polymer Structure is distinct from polymerization process Chemistry 367 L/392 N

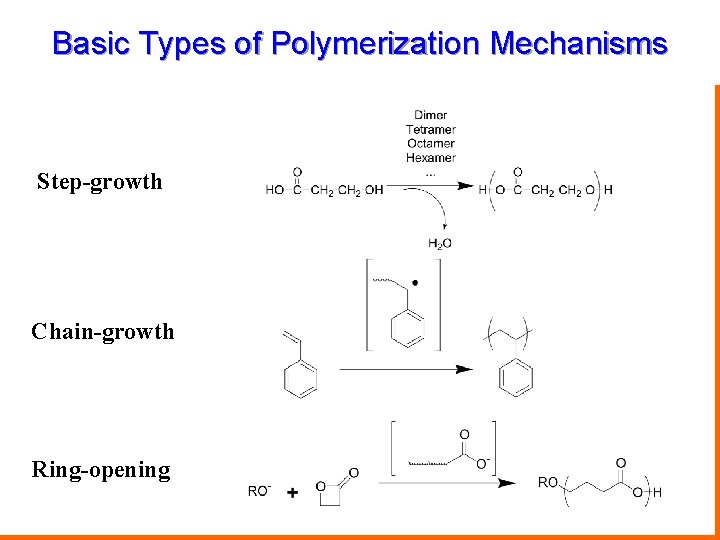
Basic Types of Polymerization Mechanisms Step-growth Chain-growth Ring-opening Chemistry 367 L/392 N

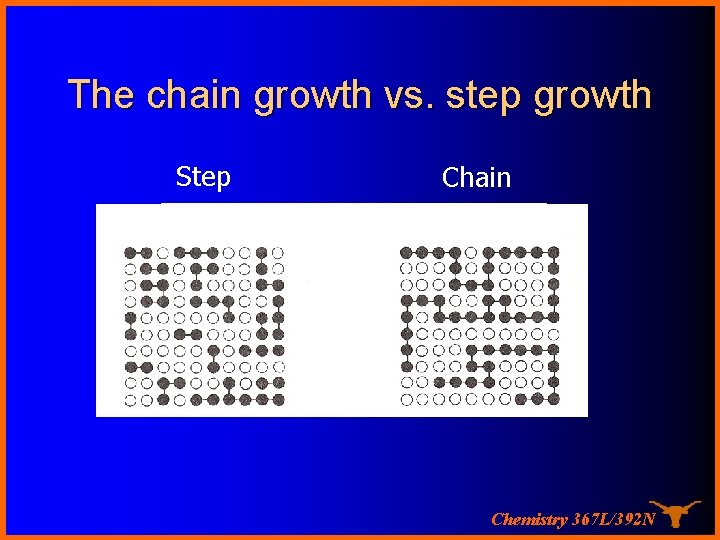
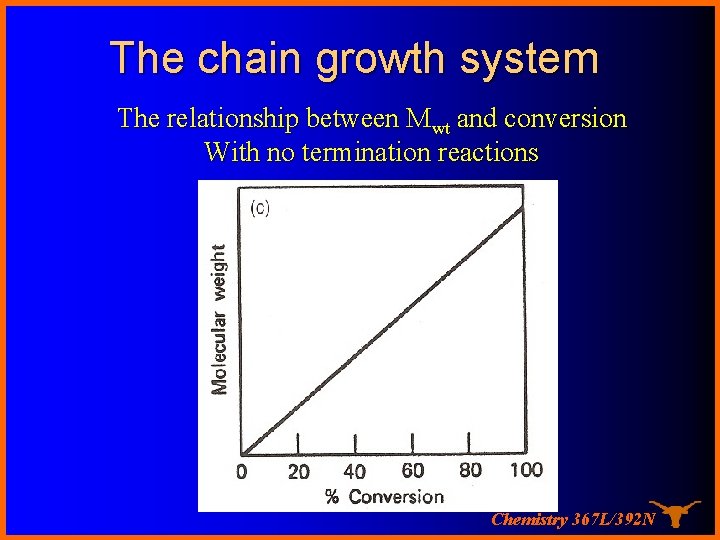
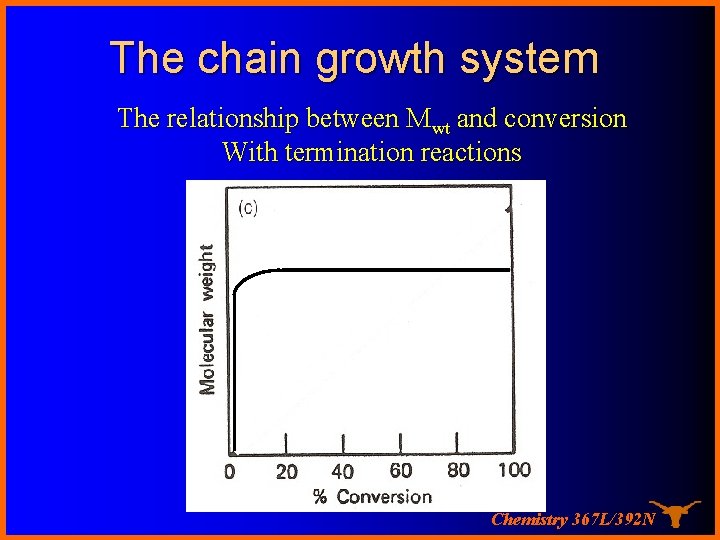
Chain growth system The characteristic of a chain polymer is that polymer growth takes place by monomer reacting only with the reactive centers. Monomer does not react with monomer and the different-sized species such as dimer, trimer, and n-mer do not react with each other. The polymerization ceases when the active center is destroyed by termination reaction(s). Chemistry 367 L/392 N

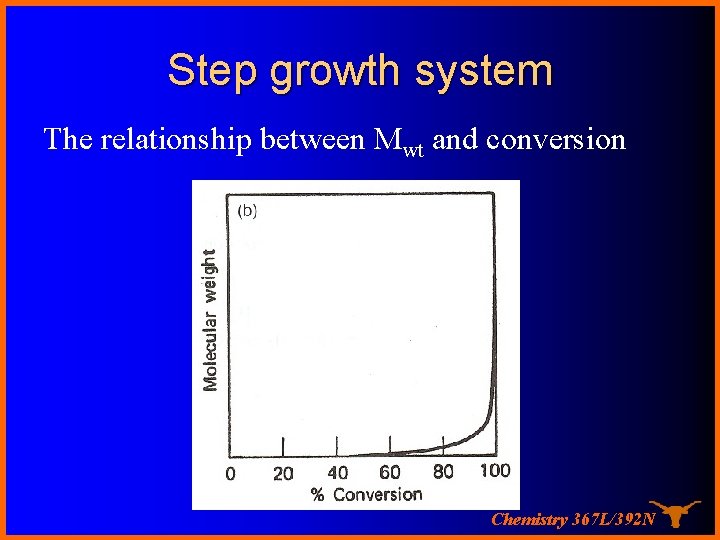
Step Growth system A condensation takes place between two polyfunctional molecules to produce one larger polyfunctional molecule with the possible elimination of a small molecule such as water. The reaction continues until one of the reagents is used up. Chemistry 367 L/392 N

The chain growth vs. step growth Step Chain Chemistry 367 L/392 N

- Step-growth polymerization Chemistry 367 L/392 N

- Chain-growth polymerization Chemistry 367 L/392 N

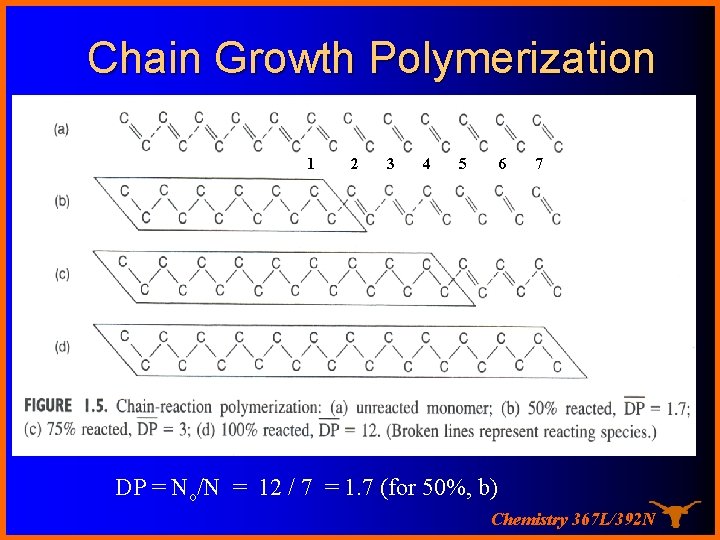
Chain Growth Polymerization 11 2 3 4 5 6 7 DP = No/N = 12 / 7 = 1. 7 (for 50%, b) Chemistry 367 L/392 N

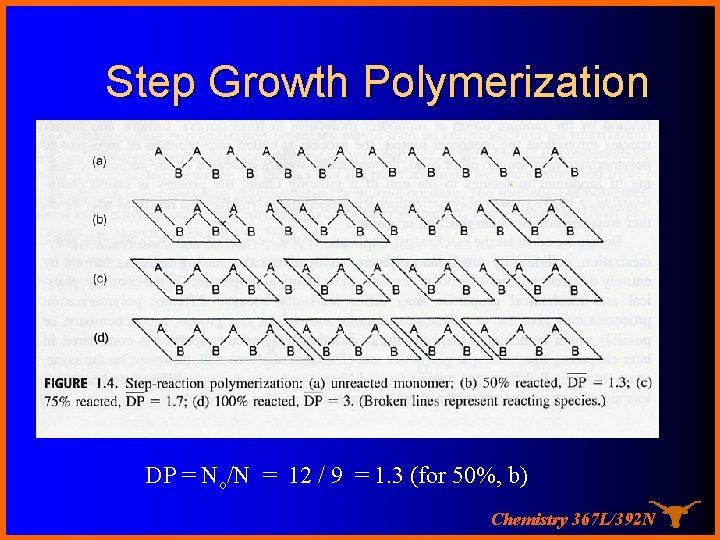
Step Growth Polymerization DP = No/N = 12 / 9 = 1. 3 (for 50%, b) Chemistry 367 L/392 N

The chain growth system The relationship between Mwt and conversion With no termination reactions Chemistry 367 L/392 N

The chain growth system The relationship between Mwt and conversion With termination reactions Chemistry 367 L/392 N

Step growth system The relationship between Mwt and conversion Chemistry 367 L/392 N

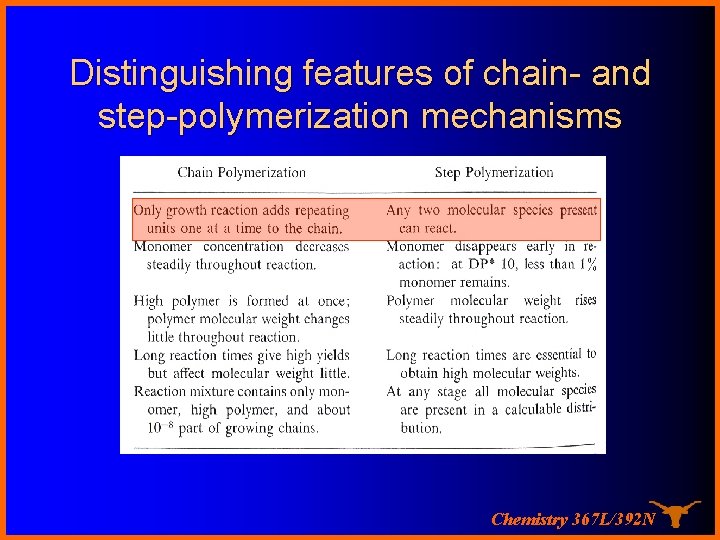
Distinguishing features of chain- and step-polymerization mechanisms Chemistry 367 L/392 N

Let’s look at this more closely…. Consider a flask of monomer …. If there are No molecules in the flask at time = 0 and N remaining at time t then the DP at time t is the average degree of polymerization… must just be N 0/N! Chemistry 367 L/392 N

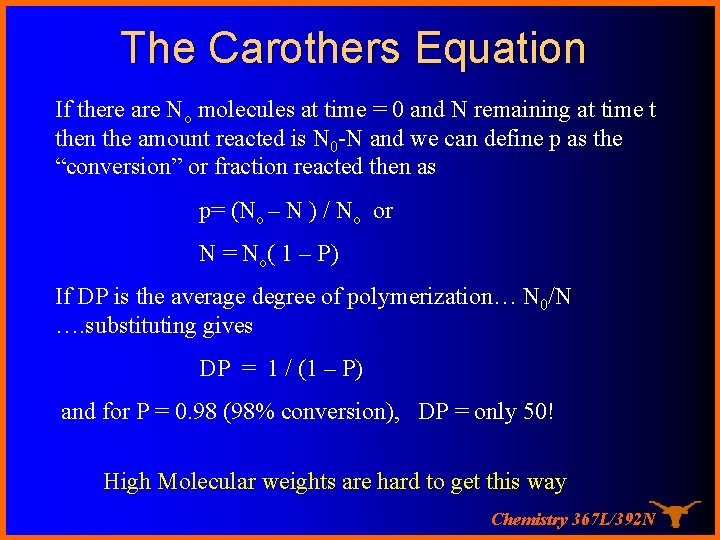
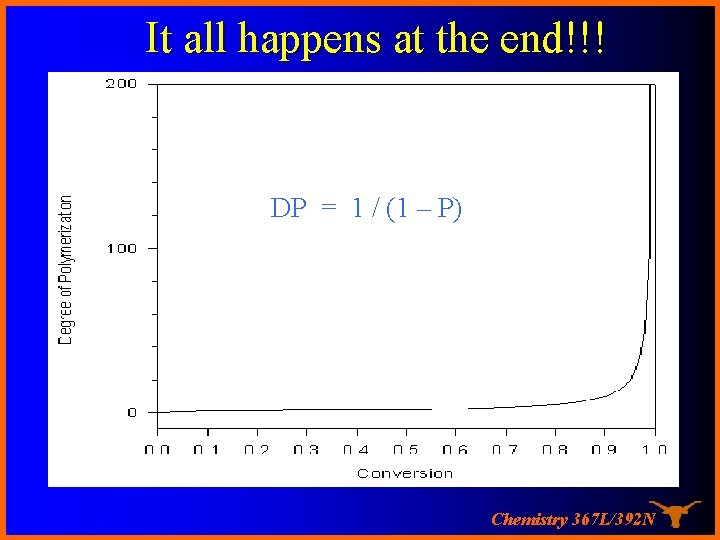
The Carothers Equation If there are No molecules at time = 0 and N remaining at time t then the amount reacted is N 0 -N and we can define p as the “conversion” or fraction reacted then as p= (No – N ) / No or N = No( 1 – P) If DP is the average degree of polymerization… N 0/N …. substituting gives DP = 1 / (1 – P) and for P = 0. 98 (98% conversion), DP = only 50! High Molecular weights are hard to get this way Chemistry 367 L/392 N

It all happens at the end!!! DP = 1 / (1 – P) Chemistry 367 L/392 N


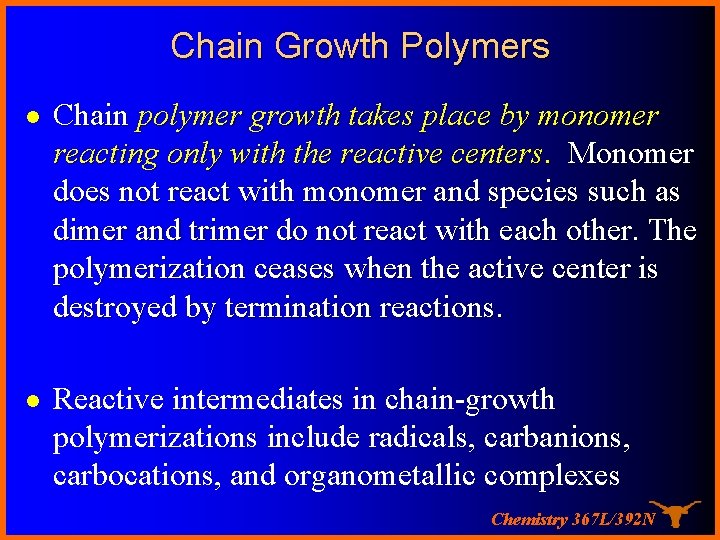
Chain Growth Polymers l Chain polymer growth takes place by monomer reacting only with the reactive centers. Monomer does not react with monomer and species such as dimer and trimer do not react with each other. The polymerization ceases when the active center is destroyed by termination reactions. l Reactive intermediates in chain-growth polymerizations include radicals, carbanions, carbocations, and organometallic complexes Chemistry 367 L/392 N

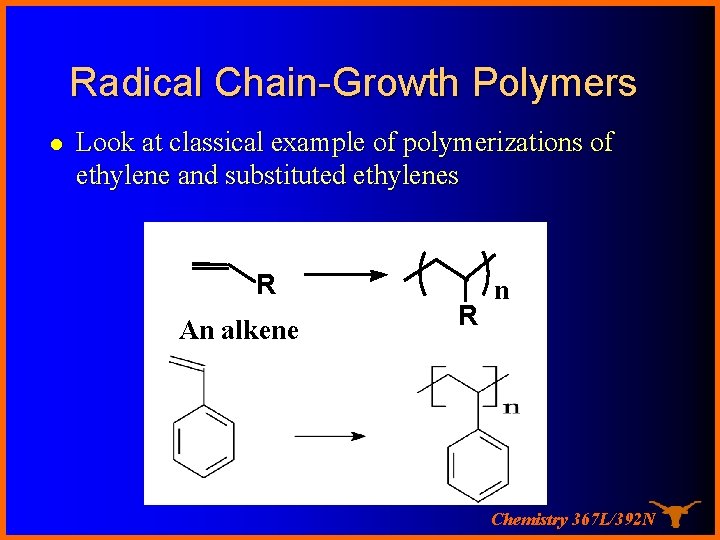
Radical Chain-Growth Polymers l Look at classical example of polymerizations of ethylene and substituted ethylenes R An alkene R n Chemistry 367 L/392 N

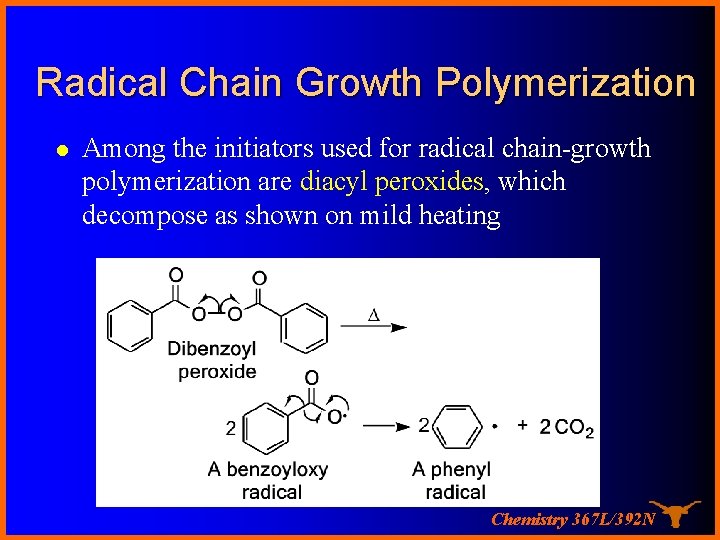
Radical Chain Growth Polymerization l Among the initiators used for radical chain-growth polymerization are diacyl peroxides, which decompose as shown on mild heating Chemistry 367 L/392 N

Benzoyl peroxide is a popular pharmaceutical Chemistry 367 L/392 N

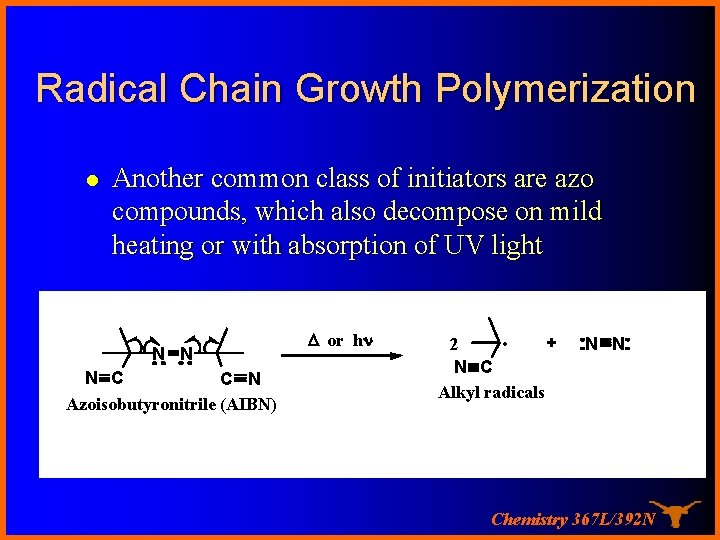
Radical Chain Growth Polymerization l Another common class of initiators are azo compounds, which also decompose on mild heating or with absorption of UV light N N N C C N Azoisobutyronitrile (AIBN) D or hn + 2 • N C Alkyl radicals N N Chemistry 367 L/392 N

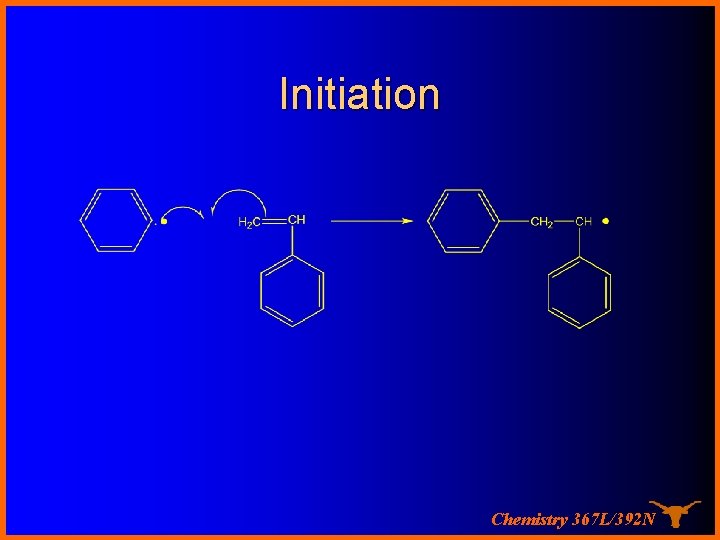
Initiation Chemistry 367 L/392 N

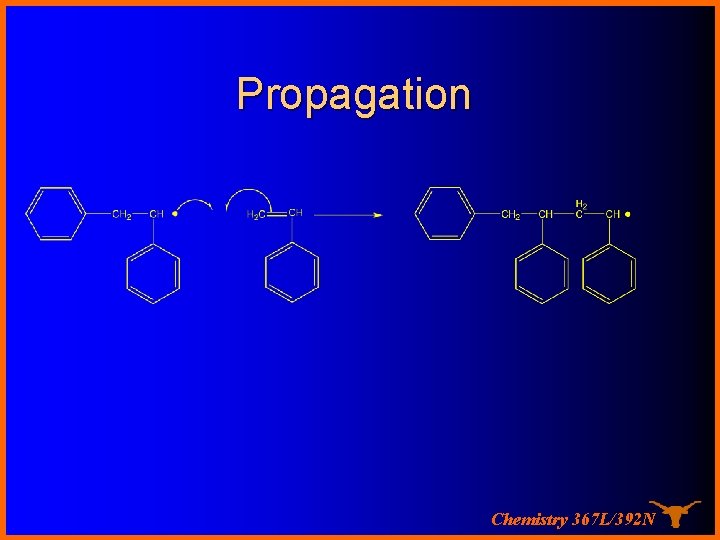
Propagation Chemistry 367 L/392 N

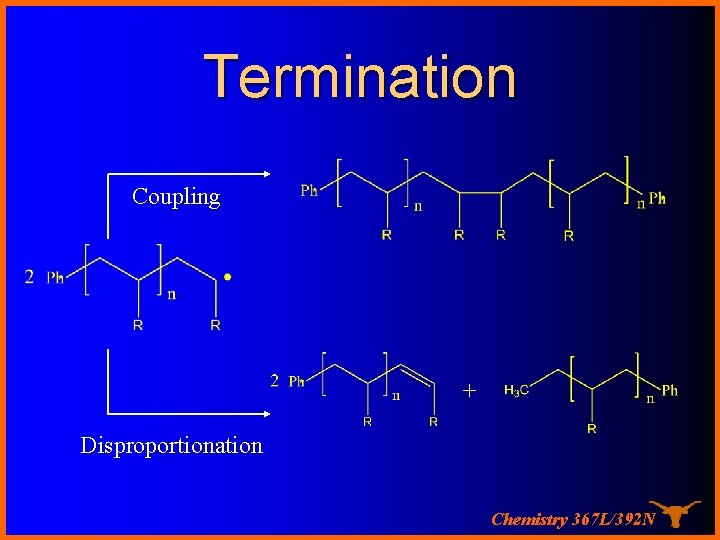
Termination Coupling + Disproportionation Chemistry 367 L/392 N