POLYMER SOLUTIONS K A U S A R



- Slides: 27

POLYMER SOLUTIONS K A U S A R A H M A D K U L L I Y Y A H O F P H A R M A C Y , I I U M H T T P : / / S T A F F. I I U. E D U. M Y / A K A U S A R Physical Pharmacy 2 1

CONTENTS • Molecular characteristics • Polymer assemblies • Polymer dissolution • Polymer swelling • Viscosity of polymer solutions • Solubility factors • Gel • Interactions in polymer solutions • Solubility parameter Physical Pharmacy 2 2

MOLECULAR CHARACTERISTICS • Polymers are dispersed as isolated molecules in very dilute solutions • molecular characteristics necessary for understanding polymer properties can be determined: • chain length, conformation, and flexibility http: //www. chem. sci. osaka-u. ac. jp/graduate/mms/English_version/Norisuye_lab. html Physical Pharmacy 2 3

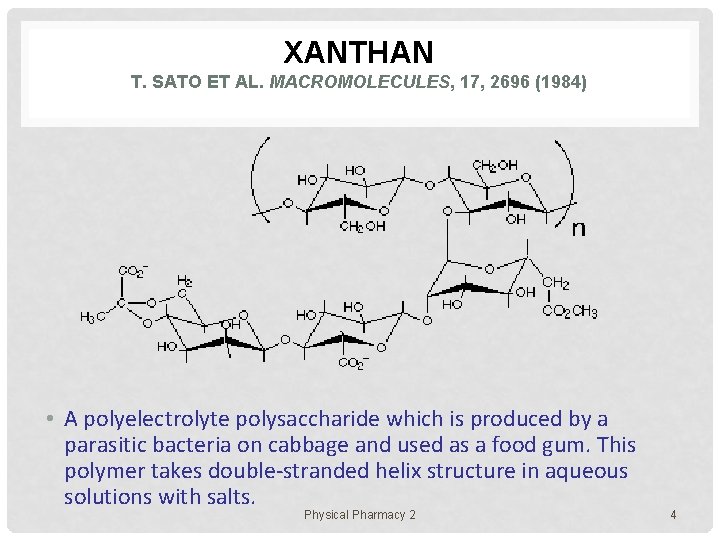
XANTHAN T. SATO ET AL. MACROMOLECULES, 17, 2696 (1984) • A polyelectrolyte polysaccharide which is produced by a parasitic bacteria on cabbage and used as a food gum. This polymer takes double-stranded helix structure in aqueous solutions with salts. Physical Pharmacy 2 4

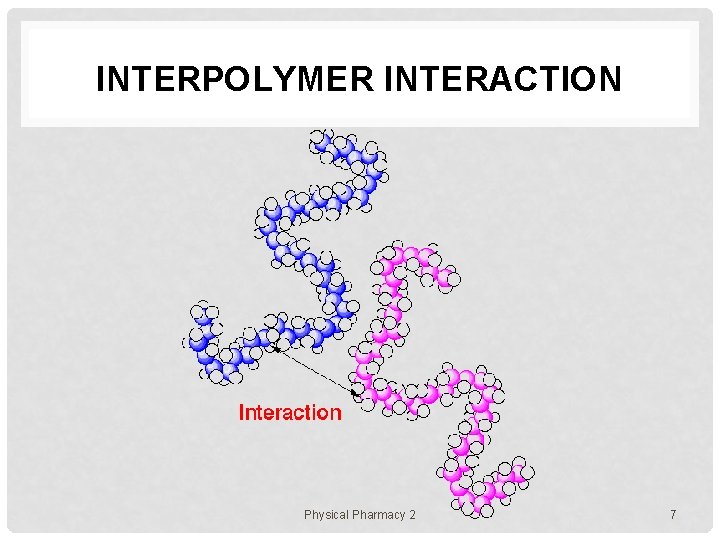
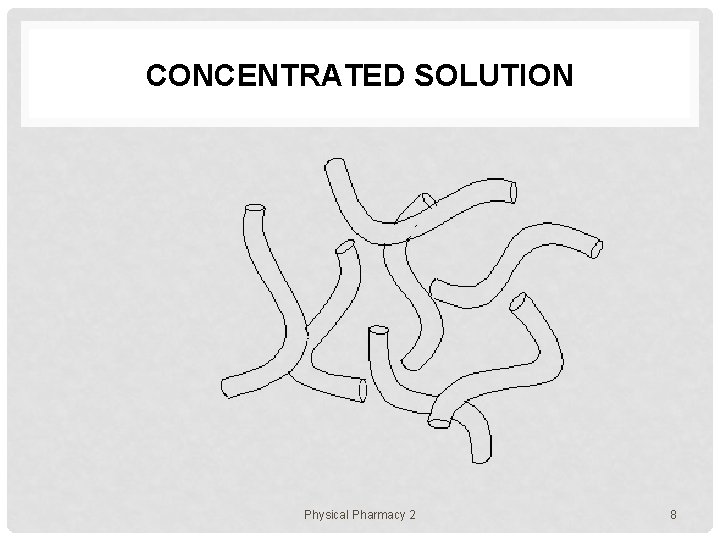
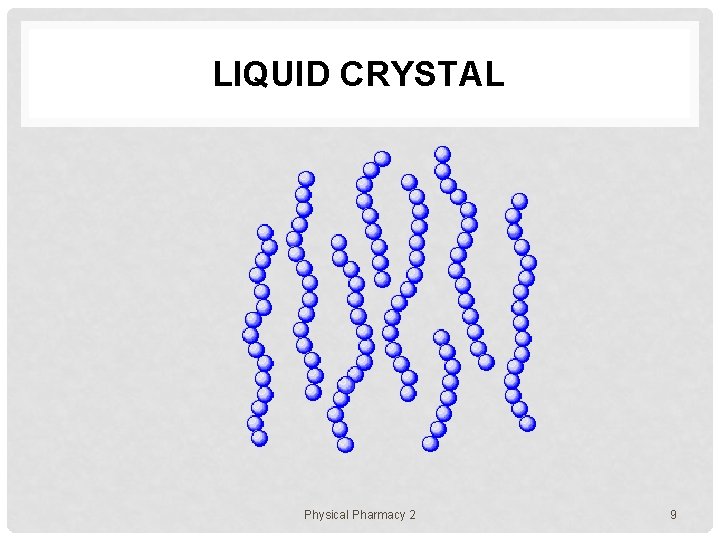
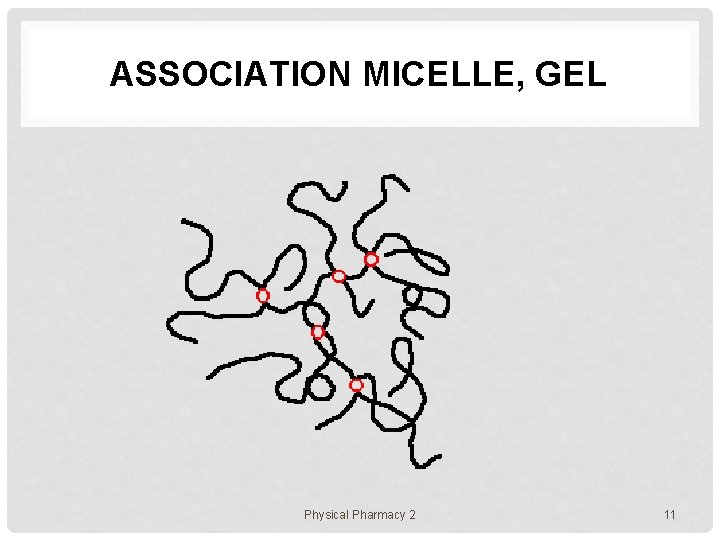
POLYMER ASSEMBLIES In solution, polymer chains exist in various states e. g. 1. uniformly molecularly dispersed state (dilute) 2. aggregating or micellar state • 3. gel state • 4. a number of polymer chains are assembled together polymer chains form a network though the system liquid crystalline state • polymer chains align toward a certain direction Physical Pharmacy 2 5

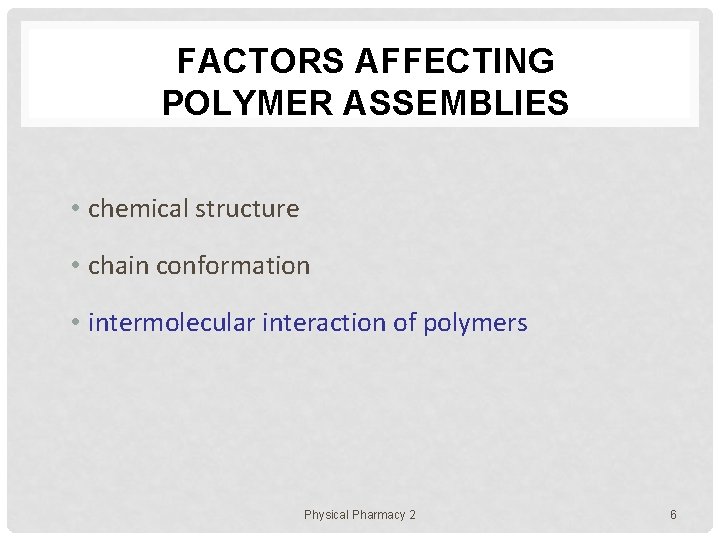
FACTORS AFFECTING POLYMER ASSEMBLIES • chemical structure • chain conformation • intermolecular interaction of polymers Physical Pharmacy 2 6

INTERPOLYMER INTERACTION Physical Pharmacy 2 7

CONCENTRATED SOLUTION Physical Pharmacy 2 8

LIQUID CRYSTAL Physical Pharmacy 2 9

Helix Physical Pharmacy 2 10

ASSOCIATION MICELLE, GEL Physical Pharmacy 2 11

AMPHIPHILIC POLYMERS Physical Pharmacy 2 12

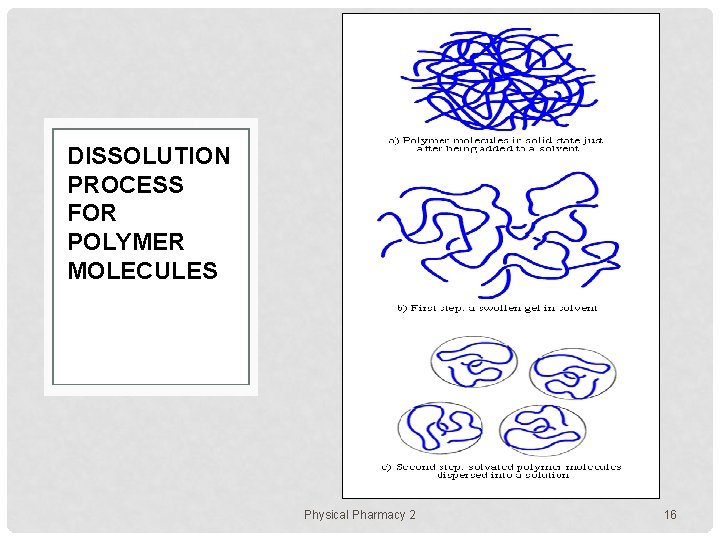
POLYMER DISSOLUTION HTTP: //WWW. PSRC. USM. EDU/MACROG/PROPERTY/SOLPOL/PS 1. HTM • the dissolution of a polymer is a slow process • due to size, structure/coiled shape, MW & the attraction forces between them, polymer molecules become dissolved quite slowly than low molecular weight molecules. • when a low MW solute such as sucrose is added to water, the dissolution process takes place almost immediately. • The sugar molecules leave the crystal lattice progressively, disperse in water, and form a solution. Physical Pharmacy 2 13

POLYMER DISSOLUTION PROCESS • Billmeyer Jr. (1975) points out that there are two stages involved in this process: 1. polymer swelling 2. dissolution Physical Pharmacy 2 14

POLYMER SWELLING When a polymer is added to a given solvent, attraction as well as dispersion forces begin acting between its segments, according to their polarity, chemical characteristics, and solubility parameter. If the polymer-solvent interactions >> polymer attraction forces, the chain segment start to absorb solvent molecules, increasing the volume of the polymer matrix, and loosening out from their coiled shape the segments are now "solvated" instead of "aggregated", as they were in the solid state. Thus swollen. Physical Pharmacy 2 15

DISSOLUTION PROCESS FOR POLYMER MOLECULES Physical Pharmacy 2 16

VISCOSITY OF POLYMER SOLUTIONS For small molecular size, the solute does not swell when dissolving. Since molecular mobility is not restricted, and therefore intermolecular friction does not increase drastically, the viscosity of the solvent and the solution are similar. In the dissolution process of polymers, such big molecules swell appreciably, restricting their mobility, and consequently the intermolecular friction increases. The solution in these cases, becomes highly viscous. THUS VISCOSITY MODIFIER! Physical Pharmacy 2 17

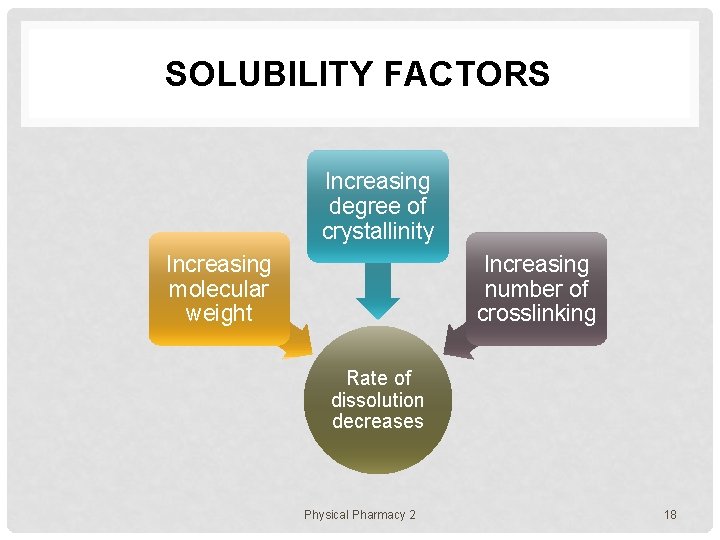
SOLUBILITY FACTORS Increasing degree of crystallinity Increasing molecular weight Increasing number of crosslinking Rate of dissolution decreases Physical Pharmacy 2 18

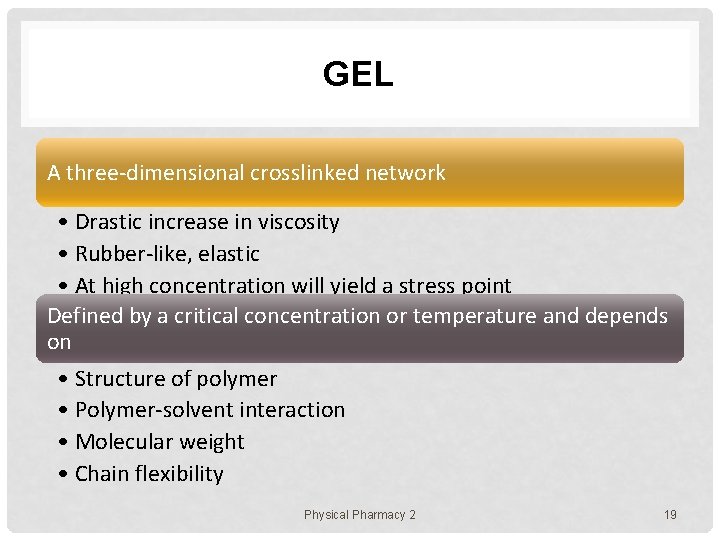
GEL A three-dimensional crosslinked network • Drastic increase in viscosity • Rubber-like, elastic • At high concentration will yield a stress point Defined by a critical concentration or temperature and depends on • Structure of polymer • Polymer-solvent interaction • Molecular weight • Chain flexibility Physical Pharmacy 2 19

TYPE 1 GEL Irreversible formation of gel as a result of network formed by covalent crosslinking Used in drug delivery • Poly(glycol methacrylate) implant • Surgical suture with gel coating • Softlens • Eye medication via viscosity control Physical Pharmacy 2 20

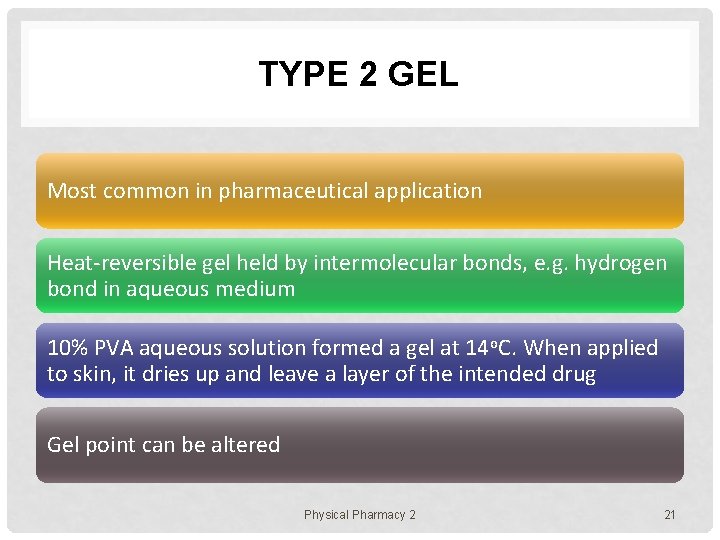
TYPE 2 GEL Most common in pharmaceutical application Heat-reversible gel held by intermolecular bonds, e. g. hydrogen bond in aqueous medium 10% PVA aqueous solution formed a gel at 14 o. C. When applied to skin, it dries up and leave a layer of the intended drug Gel point can be altered Physical Pharmacy 2 21

HETEROGELS • A copolymer A-B may exhibit different structure • when immersed in different solvents • depending of the swellability of the composites A and B towards the solvent Physical Pharmacy 2 22

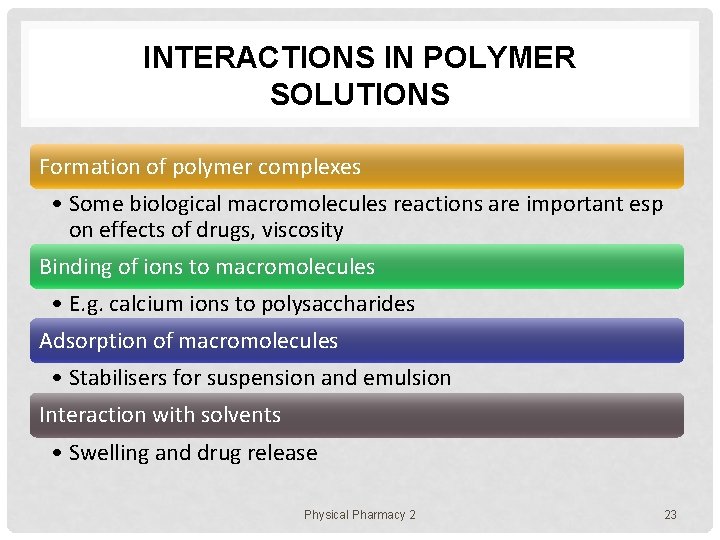
INTERACTIONS IN POLYMER SOLUTIONS Formation of polymer complexes • Some biological macromolecules reactions are important esp on effects of drugs, viscosity Binding of ions to macromolecules • E. g. calcium ions to polysaccharides Adsorption of macromolecules • Stabilisers for suspension and emulsion Interaction with solvents • Swelling and drug release Physical Pharmacy 2 23

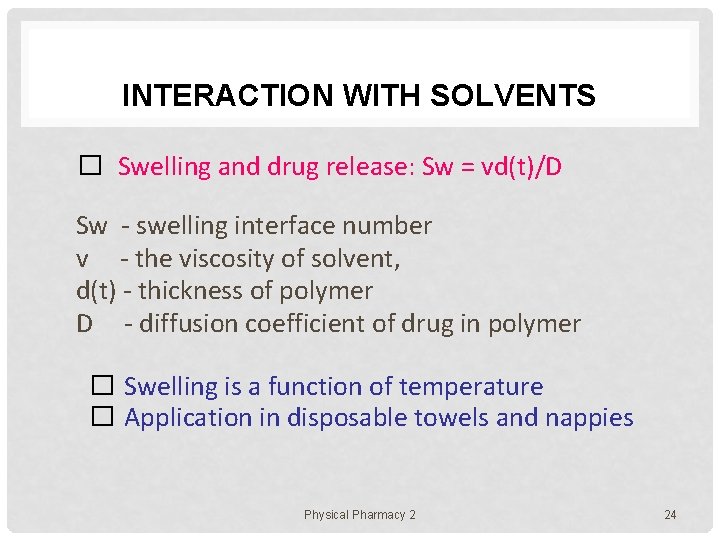
INTERACTION WITH SOLVENTS � Swelling and drug release: Sw = vd(t)/D Sw - swelling interface number v - the viscosity of solvent, d(t) - thickness of polymer D - diffusion coefficient of drug in polymer � Swelling is a function of temperature � Application in disposable towels and nappies Physical Pharmacy 2 24

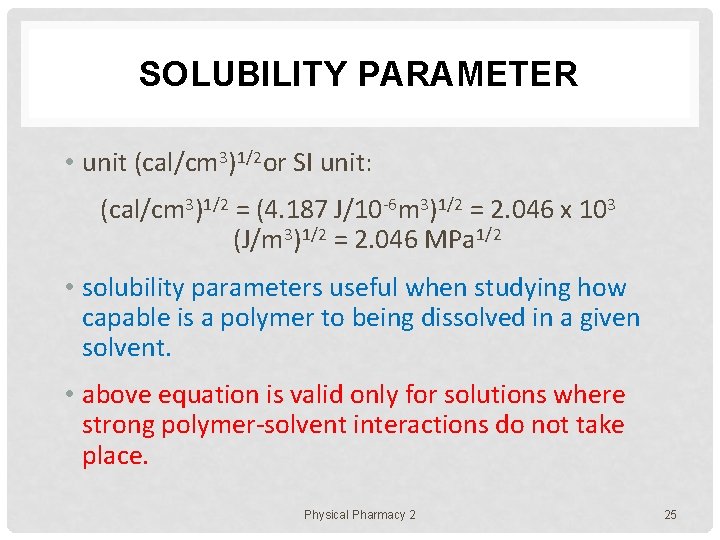
SOLUBILITY PARAMETER • unit (cal/cm 3)1/2 or SI unit: (cal/cm 3)1/2 = (4. 187 J/10 -6 m 3)1/2 = 2. 046 x 103 (J/m 3)1/2 = 2. 046 MPa 1/2 • solubility parameters useful when studying how capable is a polymer to being dissolved in a given solvent. • above equation is valid only for solutions where strong polymer-solvent interactions do not take place. Physical Pharmacy 2 25

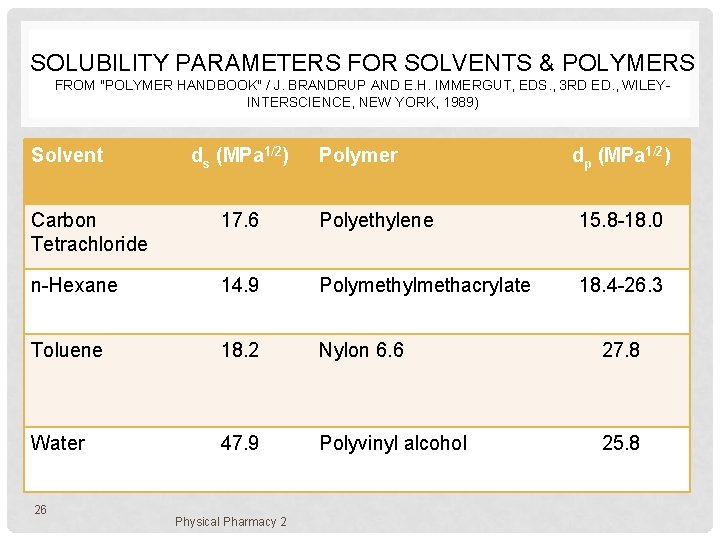
SOLUBILITY PARAMETERS FOR SOLVENTS & POLYMERS FROM "POLYMER HANDBOOK" / J. BRANDRUP AND E. H. IMMERGUT, EDS. , 3 RD ED. , WILEYINTERSCIENCE, NEW YORK, 1989) Solvent ds (MPa 1/2) Polymer dp (MPa 1/2) Carbon Tetrachloride 17. 6 Polyethylene 15. 8 -18. 0 n-Hexane 14. 9 Polymethylmethacrylate 18. 4 -26. 3 Toluene 18. 2 Nylon 6. 6 27. 8 Water 47. 9 Polyvinyl alcohol 25. 8 26 Physical Pharmacy 2

REFERENCES Aulton, M. E. (1988). Pharmaceutics: The Science of dosage form design. London: Churchill Livingstone. Chasin, M & Langer, R (1990). Biodegradable polymers as drug delivery systems. New York: Marcel Dekker. Florence, A. T. & Attwood, D. (1988). Physicochemical Principles of Pharmacy (2 nd ed. ). London: Chapman & Hall Martin, A. N. (1993). Physical Pharmacy: Physical chemistry principles in Pharmaceutical Science (4 th ed. ). Philadelphia: Lea & Febiger. Vyas, S. P & Khar, R. K. (2002). Targeted and controlled drug delivery. New Delhi: CBS. Wise, D. L. (2000). Handbook of Pharmaceutical Controlled Release Technology. New York: Marcel Dekker. Physical Pharmacy 2 27