Chapter 4 Polymer Structures Polymer molecules are very

- Slides: 18

Chapter 4: Polymer Structures • Polymer molecules are very large, and primarily with strong covalent bonds. • In a solid polymer, the molecules are held together by entanglement and van der Waals forces. • Polymer solids tend to be mostly or entirely amorphous. • In this chapter, we deal with polymers made from organic compounds, which are the most common. • Cover molecular structures, molecular weight, crystallinity. • Typical properties of solids made from organic polymers: low density, low strength, inexpensive, unable to withstand high temperature. • But strength-to-weight ratio may be high, particularly for fibers. • On-line information: – General: http: //en. wikipedia. org/wiki/Polymer – History: http: //cen. acs. org/articles/91/i 36/Plastic-Planet. html – Biopolymers: http: //en. wikipedia. org/wiki/Biopolymer – Silicones (based on Si): http: //en. wikipedia. org/wiki/Silicone Last revised January 31, 2014 by W. R. Wilcox, Clarkson University.

Applications of organic polymers • Originally, natural polymers were used. For example: – Wood – Rubber – Cotton – Wool – Leather – Silk • Oldest known uses: – Building materials – Clothing – Rubber balls used by Mesoamericans 3, 600 years ago – Amber used for jewelry 13, 000 years ago • Applications for synthetic organic polymers are increasing: – When low weight is required – Often strengthened by addition of fibers, typically glass or carbon (composites) – Automobiles, aircraft, even jet engine components 2

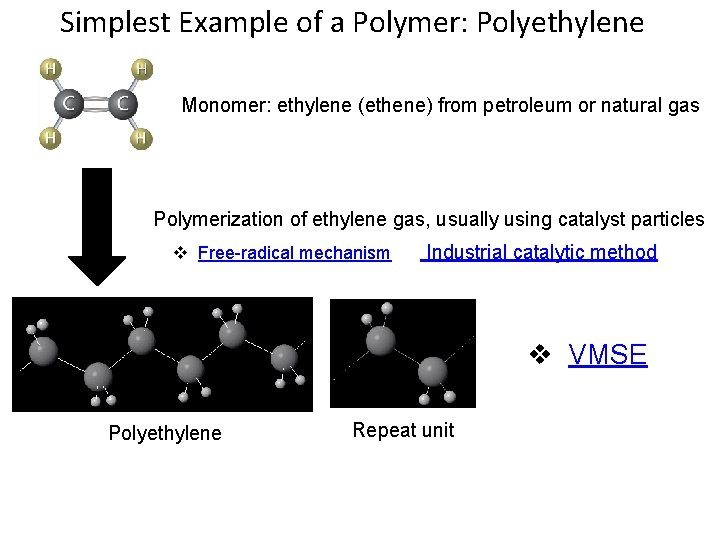
Simplest Example of a Polymer: Polyethylene Monomer: ethylene (ethene) from petroleum or natural gas Polymerization of ethylene gas, usually using catalyst particles Free-radical mechanism Industrial catalytic method VMSE Polyethylene Repeat unit

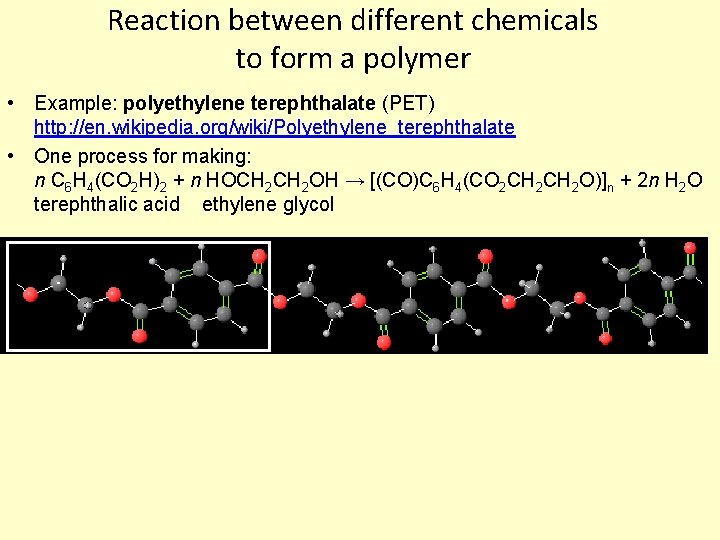
Reaction between different chemicals to form a polymer • Example: polyethylene terephthalate (PET) http: //en. wikipedia. org/wiki/Polyethylene_terephthalate • One process for making: n C 6 H 4(CO 2 H)2 + n HOCH 2 OH → [(CO)C 6 H 4(CO 2 CH 2 O)]n + 2 n H 2 O terephthalic acid ethylene glycol

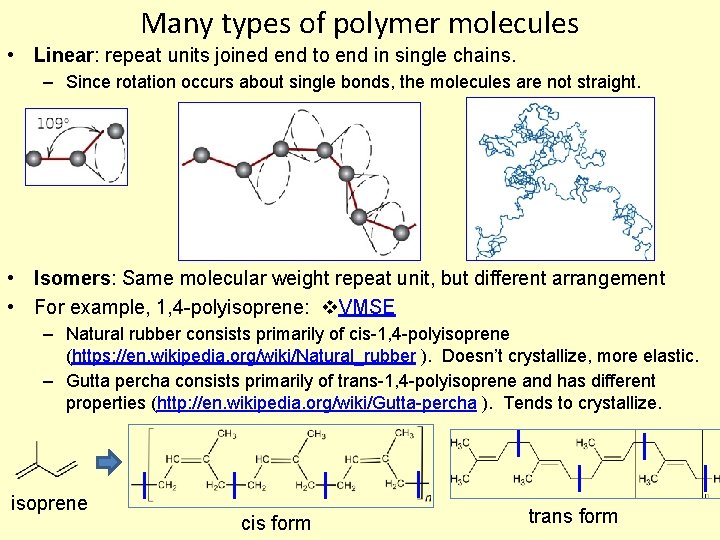
Many types of polymer molecules • Linear: repeat units joined end to end in single chains. – Since rotation occurs about single bonds, the molecules are not straight. • Isomers: Same molecular weight repeat unit, but different arrangement • For example, 1, 4 -polyisoprene: VMSE – Natural rubber consists primarily of cis-1, 4 -polyisoprene (https: //en. wikipedia. org/wiki/Natural_rubber ). Doesn’t crystallize, more elastic. – Gutta percha consists primarily of trans-1, 4 -polyisoprene and has different properties (http: //en. wikipedia. org/wiki/Gutta-percha ). Tends to crystallize. isoprene cis form trans form

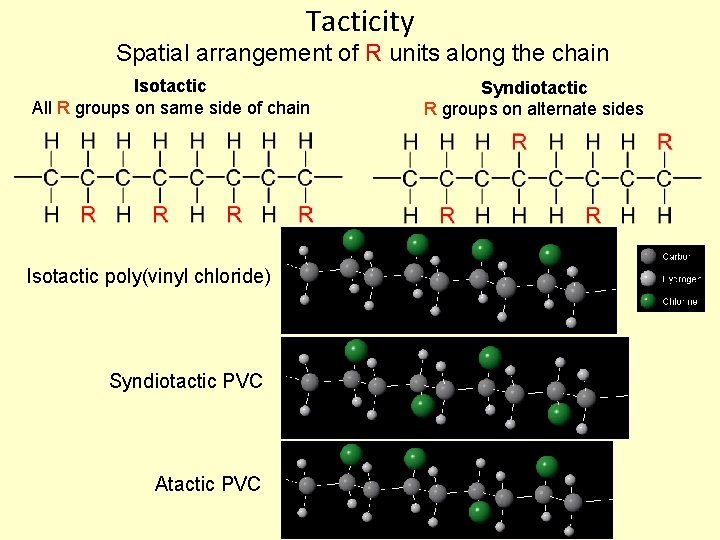
Tacticity Spatial arrangement of R units along the chain Isotactic All R groups on same side of chain Isotactic poly(vinyl chloride) Syndiotactic PVC Atactic PVC Syndiotactic R groups on alternate sides

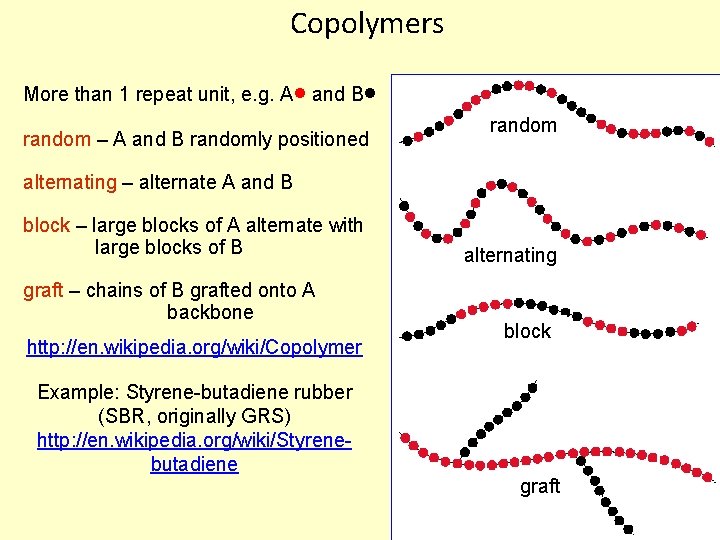
Copolymers More than 1 repeat unit, e. g. A and B random – A and B randomly positioned random alternating – alternate A and B block – large blocks of A alternate with large blocks of B graft – chains of B grafted onto A backbone http: //en. wikipedia. org/wiki/Copolymer Example: Styrene-butadiene rubber (SBR, originally GRS) http: //en. wikipedia. org/wiki/Styrenebutadiene alternating block graft

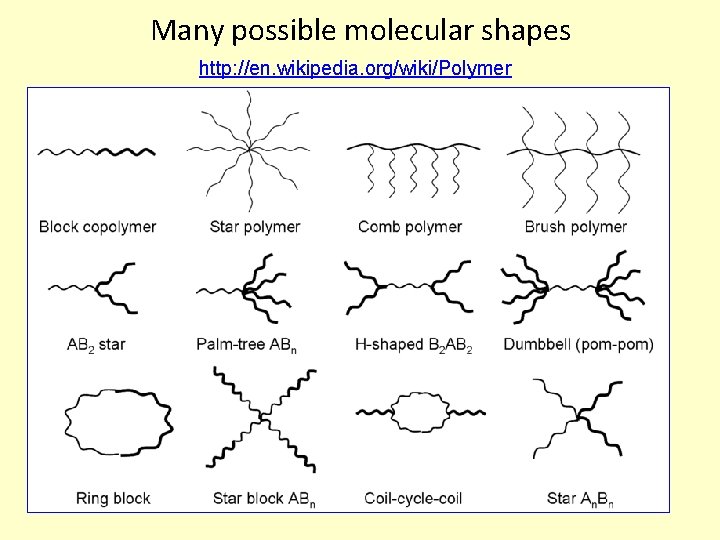
Many possible molecular shapes http: //en. wikipedia. org/wiki/Polymer

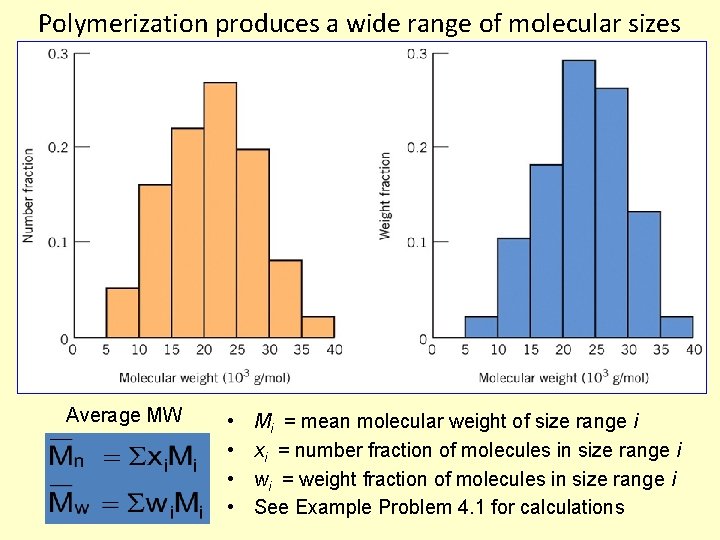
Polymerization produces a wide range of molecular sizes Average MW • • Mi = mean molecular weight of size range i xi = number fraction of molecules in size range i wi = weight fraction of molecules in size range i See Example Problem 4. 1 for calculations

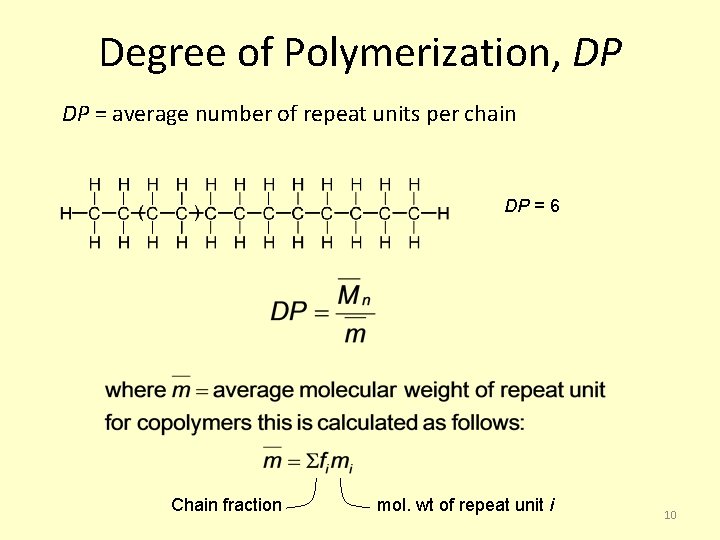
Degree of Polymerization, DP DP = average number of repeat units per chain DP = 6 Chain fraction mol. wt of repeat unit i 10

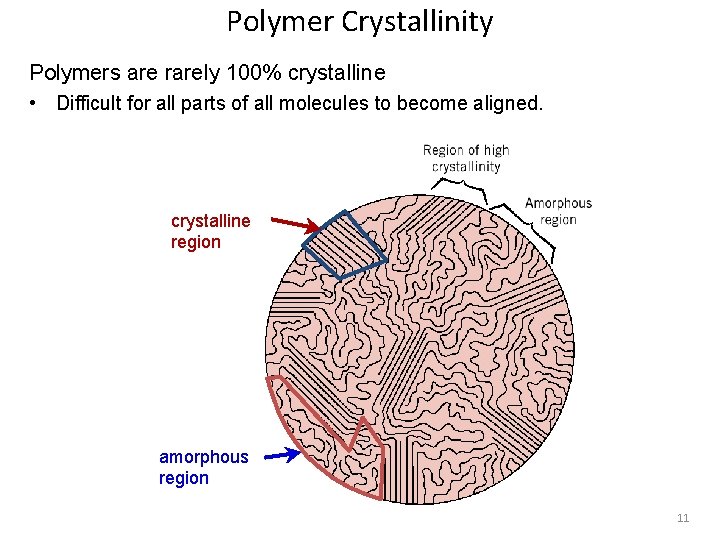
Polymer Crystallinity Polymers are rarely 100% crystalline • Difficult for all parts of all molecules to become aligned. crystalline region amorphous region 11

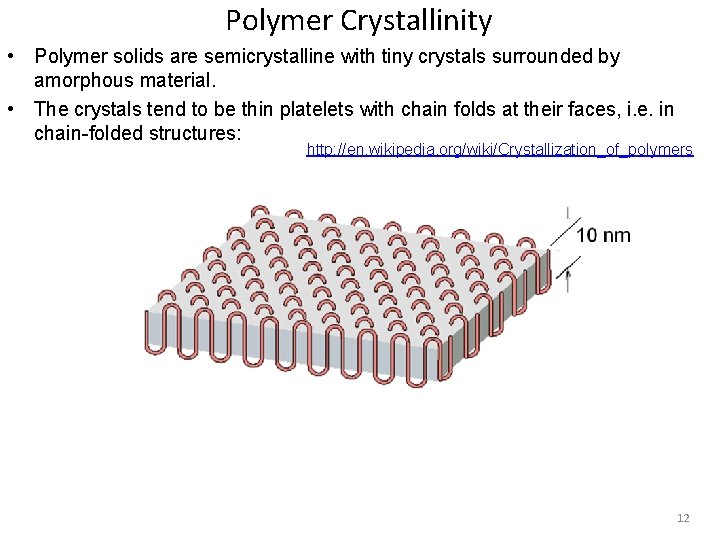
Polymer Crystallinity • Polymer solids are semicrystalline with tiny crystals surrounded by amorphous material. • The crystals tend to be thin platelets with chain folds at their faces, i. e. in chain-folded structures: http: //en. wikipedia. org/wiki/Crystallization_of_polymers 12

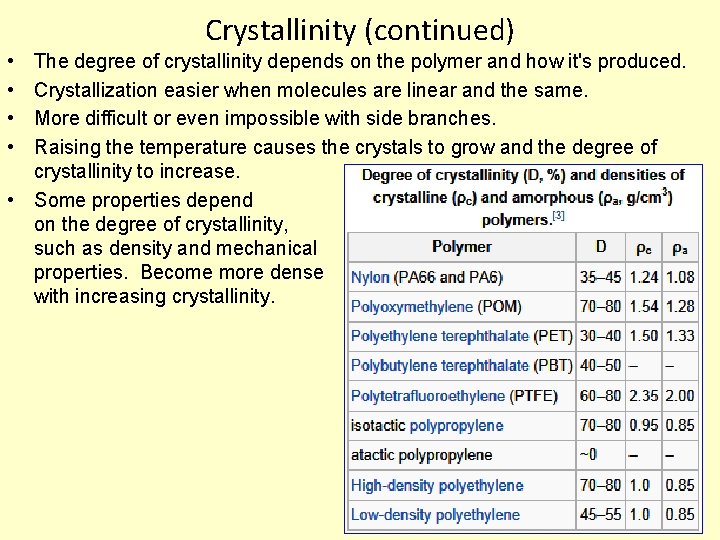
Crystallinity (continued) • • The degree of crystallinity depends on the polymer and how it's produced. Crystallization easier when molecules are linear and the same. More difficult or even impossible with side branches. Raising the temperature causes the crystals to grow and the degree of crystallinity to increase. • Some properties depend on the degree of crystallinity, such as density and mechanical properties. Become more dense with increasing crystallinity.

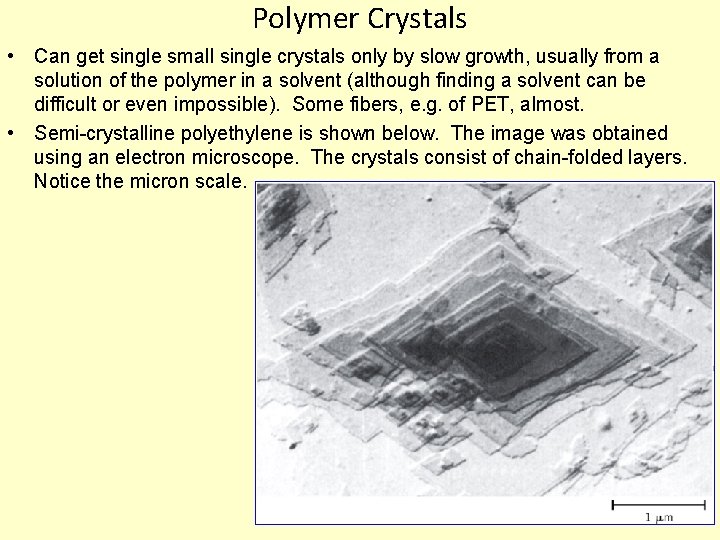
Polymer Crystals • Can get single small single crystals only by slow growth, usually from a solution of the polymer in a solvent (although finding a solvent can be difficult or even impossible). Some fibers, e. g. of PET, almost. • Semi-crystalline polyethylene is shown below. The image was obtained using an electron microscope. The crystals consist of chain-folded layers. Notice the micron scale. 14

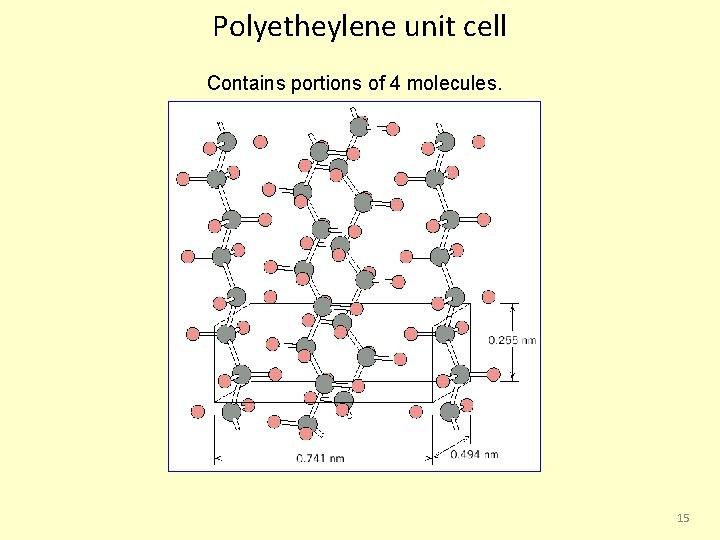
Polyetheylene unit cell Contains portions of 4 molecules. 15

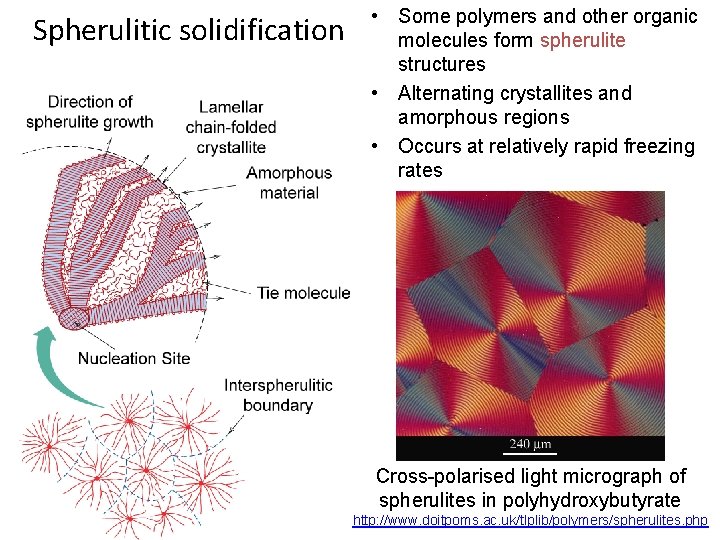
Spherulitic solidification • Some polymers and other organic molecules form spherulite structures • Alternating crystallites and amorphous regions • Occurs at relatively rapid freezing rates Cross-polarised light micrograph of spherulites in polyhydroxybutyrate http: //www. doitpoms. ac. uk/tlplib/polymers/spherulites. php

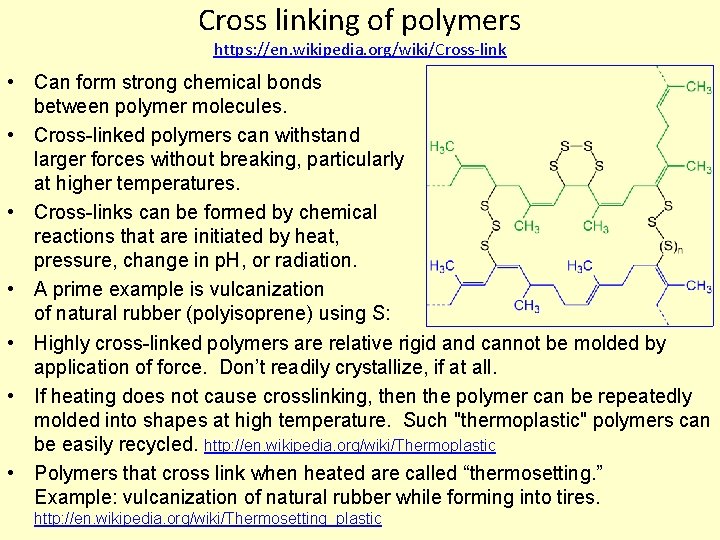
Cross linking of polymers https: //en. wikipedia. org/wiki/Cross-link • Can form strong chemical bonds between polymer molecules. • Cross-linked polymers can withstand larger forces without breaking, particularly at higher temperatures. • Cross-links can be formed by chemical reactions that are initiated by heat, pressure, change in p. H, or radiation. • A prime example is vulcanization of natural rubber (polyisoprene) using S: • Highly cross-linked polymers are relative rigid and cannot be molded by application of force. Don’t readily crystallize, if at all. • If heating does not cause crosslinking, then the polymer can be repeatedly molded into shapes at high temperature. Such "thermoplastic" polymers can be easily recycled. http: //en. wikipedia. org/wiki/Thermoplastic • Polymers that cross link when heated are called “thermosetting. ” Example: vulcanization of natural rubber while forming into tires. http: //en. wikipedia. org/wiki/Thermosetting_plastic

Summary • An unlimited number of types of polymers possible, with new ones being developed all the time. • The polymer produced in a chemical reaction depends on: – – – Composition of the reaction mixture. Catalyst used. Temperature. Pressure. Time. • Many additives used to modify the final products: fibers, particles, plasticizers, etc. Applications: http: //en. wikipedia. org/wiki/List_of_synthetic_polymers VMSE: http: //higheredbcs. wiley. com/legacy/college/callister/1118061608/vmse/mer. htm Recycling: http: //en. wikipedia. org/wiki/Plastic_recycling