Polymer Properties Exercise 4 Viscoelasticity and rheology Effect



- Slides: 18

Polymer Properties Exercise 4

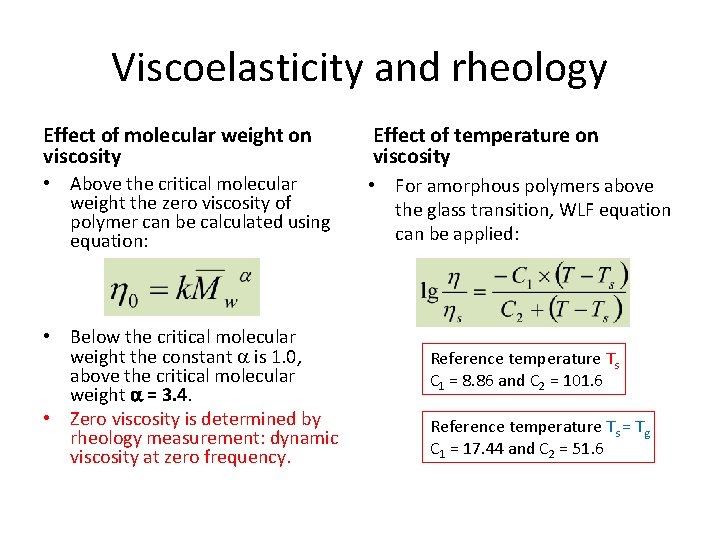
Viscoelasticity and rheology Effect of molecular weight on viscosity Effect of temperature on viscosity • Above the critical molecular weight the zero viscosity of polymer can be calculated using equation: • For amorphous polymers above the glass transition, WLF equation can be applied: • Below the critical molecular weight the constant is 1. 0, above the critical molecular weight = 3. 4. • Zero viscosity is determined by rheology measurement: dynamic viscosity at zero frequency. Reference temperature Ts C 1 = 8. 86 and C 2 = 101. 6 Reference temperature Ts = Tg C 1 = 17. 44 and C 2 = 51. 6

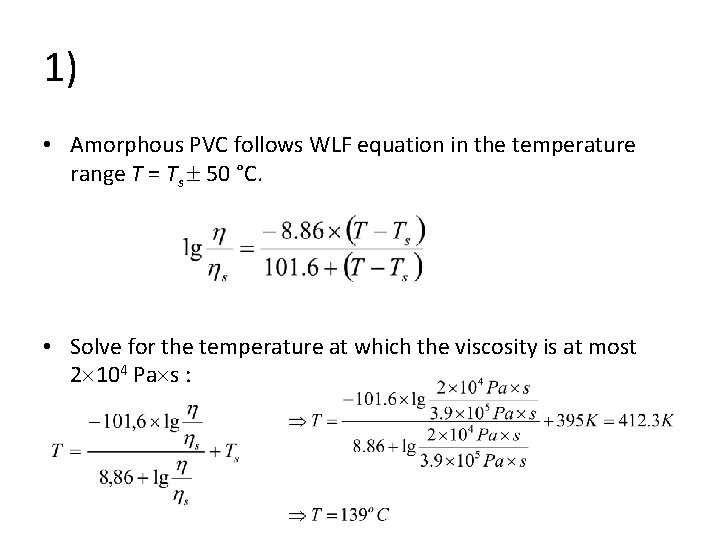
1. Viscosity • Viscosity of an amorphous PVC was measured to be 3. 9 105 Pa s at temperature 122 o. C. For processing, the viscosity should be below 2 104 Pa s, but at least 5000 Pa s. • At what temperature should the processing be done?

1) • Amorphous PVC follows WLF equation in the temperature range T = Ts 50 °C. • Solve for the temperature at which the viscosity is at most 2 104 Pa s :

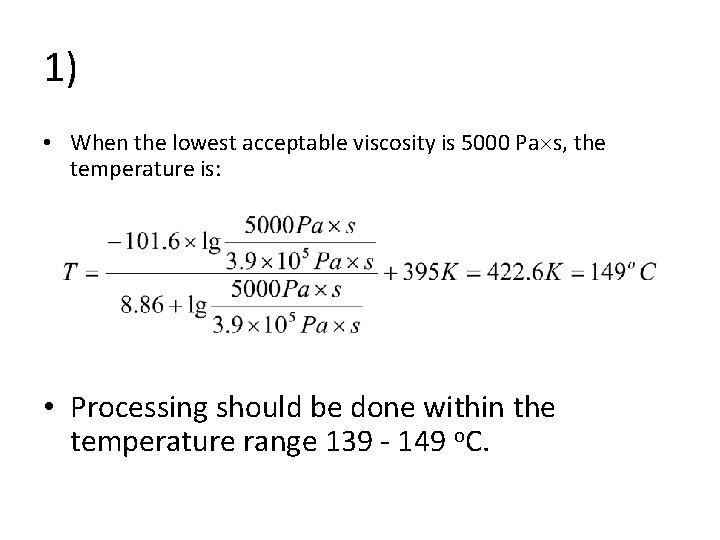
1) • When the lowest acceptable viscosity is 5000 Pa s, the temperature is: • Processing should be done within the temperature range 139 - 149 o. C.

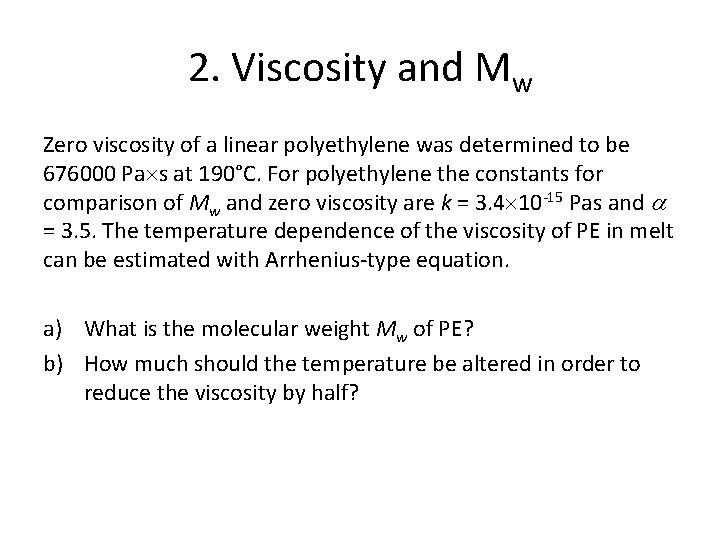
2. Viscosity and Mw Zero viscosity of a linear polyethylene was determined to be 676000 Pa s at 190°C. For polyethylene the constants for comparison of Mw and zero viscosity are k = 3. 4 10 -15 Pas and = 3. 5. The temperature dependence of the viscosity of PE in melt can be estimated with Arrhenius-type equation. a) What is the molecular weight Mw of PE? b) How much should the temperature be altered in order to reduce the viscosity by half?

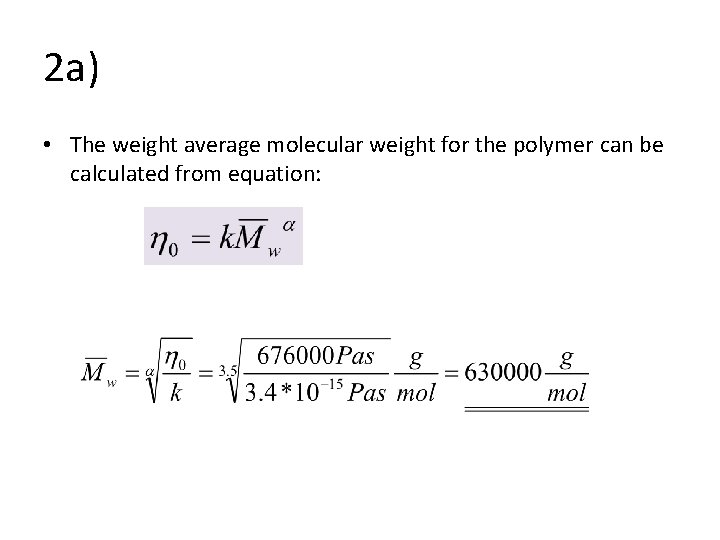
2 a) • The weight average molecular weight for the polymer can be calculated from equation:

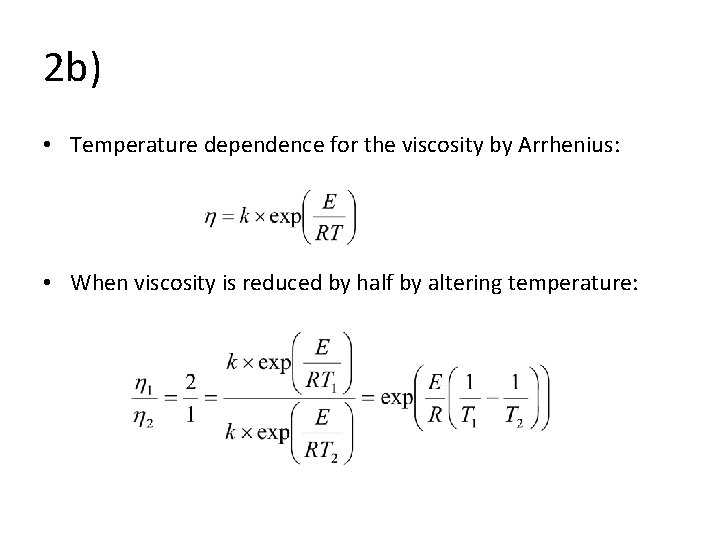
2 b) • Temperature dependence for the viscosity by Arrhenius: • When viscosity is reduced by half by altering temperature:

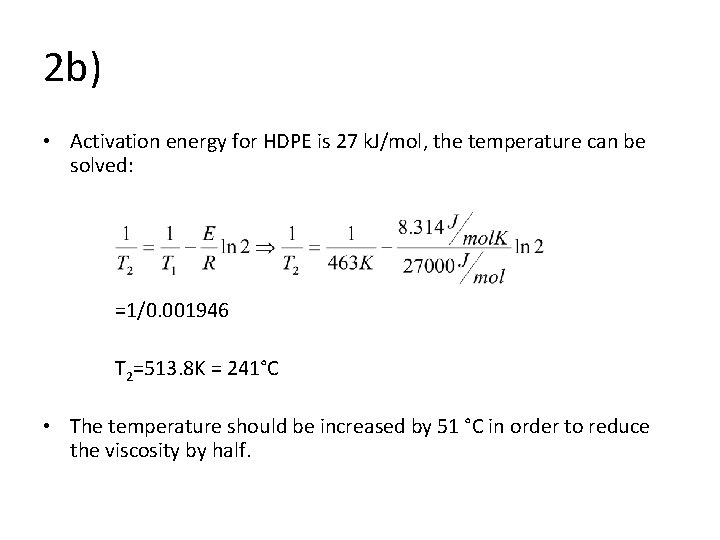
2 b) • Activation energy for HDPE is 27 k. J/mol, the temperature can be solved: =1/0. 001946 T 2=513. 8 K = 241°C • The temperature should be increased by 51 °C in order to reduce the viscosity by half.

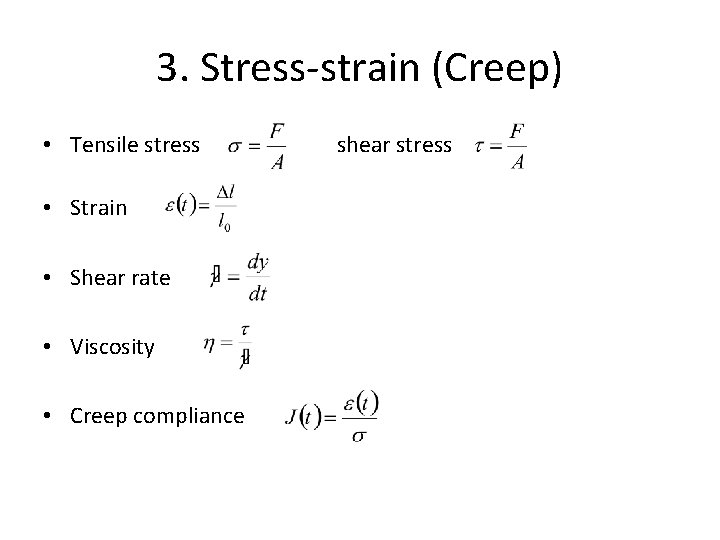
3. Stress-strain (Creep) • Tensile stress • Strain shear stress • Shear rate • Viscosity • Creep compliance

3. Creep • Polypropylene PP rod attached to the ceiling (length 200 mm, width 25. 0 mm, thickness 3. 0 mm) is loaded with 30 kg´s. How much will the polymer creep in two minutes when the creep compliance J(t) follows the equation (t is time in minutes)? J(t) = 1. 5 - exp(-t/6 min) GPa-1

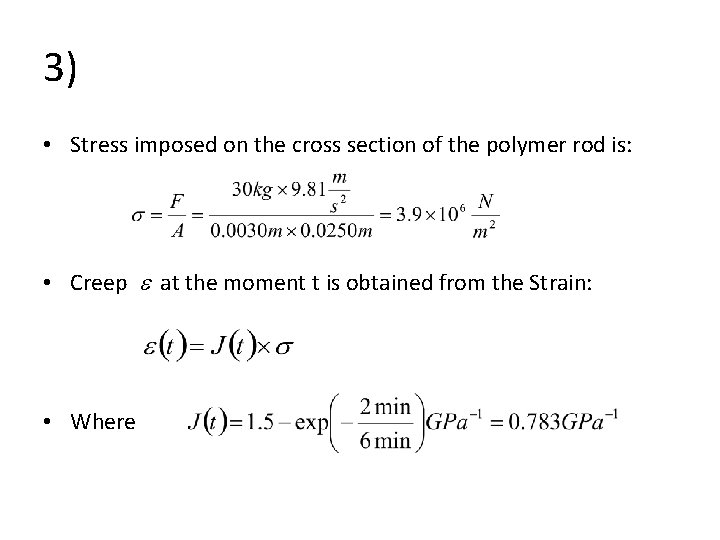
3) • Stress imposed on the cross section of the polymer rod is: • Creep at the moment t is obtained from the Strain: • Where

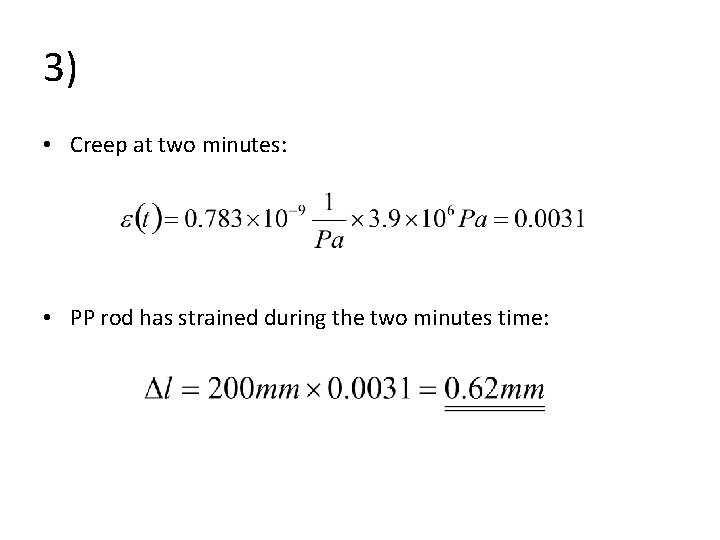
3) • Creep at two minutes: • PP rod has strained during the two minutes time:

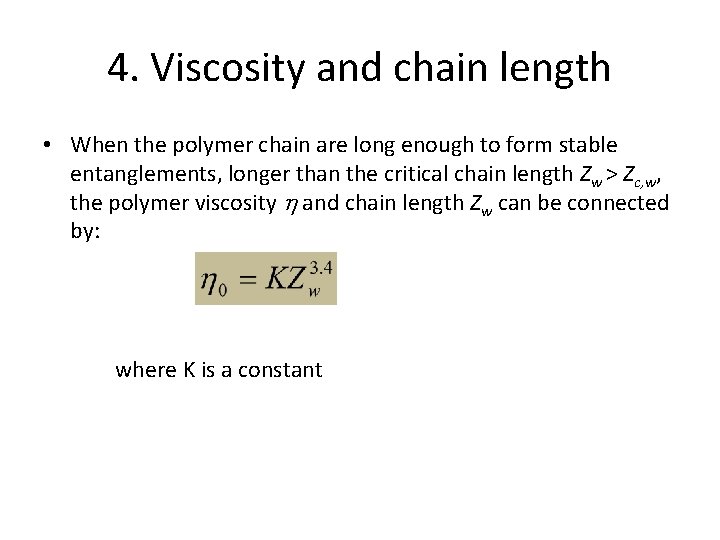
4. Viscosity and chain length • When the polymer chain are long enough to form stable entanglements, longer than the critical chain length Zw > Zc, w, the polymer viscosity and chain length Zw can be connected by: where K is a constant

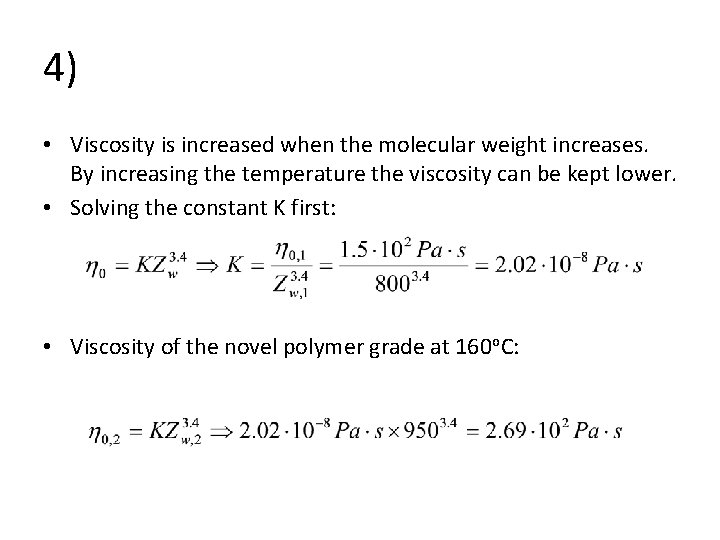
4) • The usual processing temperature of polystyrene cups is 160 o. C and the melt viscosity is then 1. 5 102 Pa s, provided that the mainchain length of PS is Zw = 800. The quality of the polymer however varies and one day the Zw = 950. Processing is tuned for a particular viscosity range. • How should the processing temperature be altered so that the melt viscosity would still be 1. 5 102 Pa s? Glass transition temperature of PS is 100 o. C.

4) • Viscosity is increased when the molecular weight increases. By increasing the temperature the viscosity can be kept lower. • Solving the constant K first: • Viscosity of the novel polymer grade at 160 o. C:

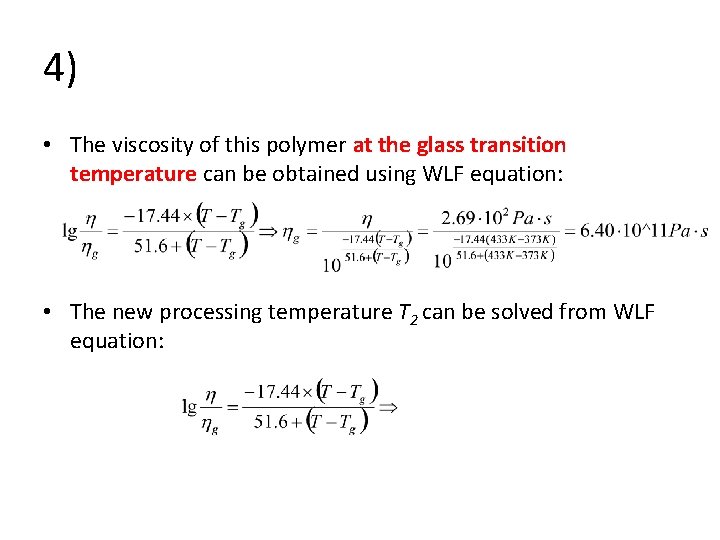
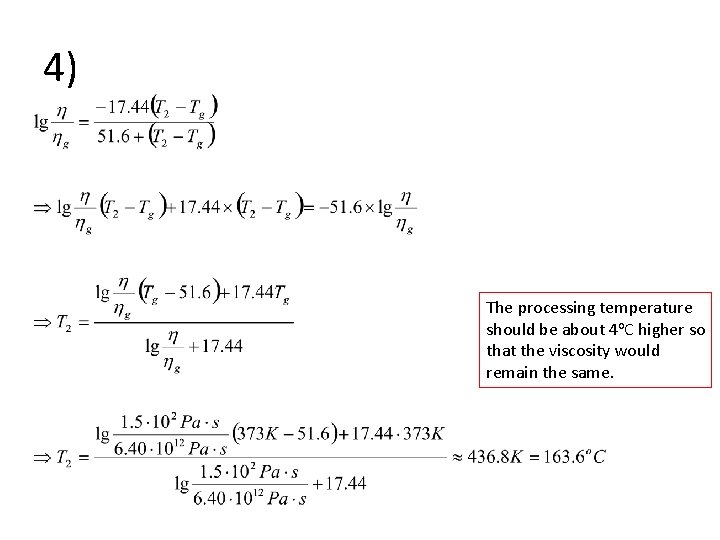
4) • The viscosity of this polymer at the glass transition temperature can be obtained using WLF equation: • The new processing temperature T 2 can be solved from WLF equation:

4) The processing temperature should be about 4 o. C higher so that the viscosity would remain the same.