Physics 124 Particles and Waves Chapter 30 Quantum









- Slides: 39

Physics 124 — Particles and Waves Chapter 30. Quantum Physics Frans Pretorius University of Alberta

The Wave-Particle Duality Ø this chapter deals with the so called wave-particle duality of matter Ø all known forms of matter (photons, electrons, muons, neutrinos, …) exhibit both particle-like and wave-like characteristics Ø even gravity is expected to exhibit this duality, though no one has yet detected the “particle” of the gravitational field, dubbed the graviton. particle-like behavior wave-like behavior come in discrete “pieces”, or quanta amplitude can be arbitrarily small when two particles collide, they scatter off one another when waves “collide”, they pass through one another, producing interference effects localized at a point in space spread out over a region of space PHYS HON, Chapter 27 -28: Quantum Theory 2

The Wave-Particle Duality Ø many interesting and sometimes puzzling consequences of this Ø the energy of a particle is proportional to the frequency of the corresponding wave Ø the momentum of a particle is proportional to the wavelength of the corresponding wave Ø these relationships require a new fundamental constant of nature: Planck’s constant Ø correctly describes many effects, including Ø blackbody radiation Ø the photoelectric effect (electrons ejected off a metal surface when light shines on it) Ø the Compton effect (a photon elastically colliding with an electron) Ø explains the stability of atoms Ø implies the Heisenberg uncertainty principle (one aspect of this is that an experiment can never discern both the momentum and position of a particle with arbitrary accuracy). Ø is one of the foundations of quantum mechanics PHYS HON, Chapter 27 -28: Quantum Theory 3

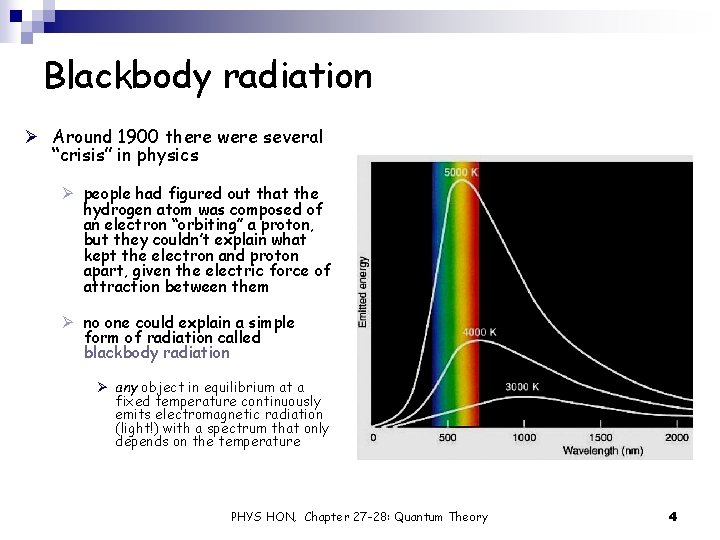
Blackbody radiation Ø Around 1900 there were several “crisis” in physics Ø people had figured out that the hydrogen atom was composed of an electron “orbiting” a proton, but they couldn’t explain what kept the electron and proton apart, given the electric force of attraction between them Ø no one could explain a simple form of radiation called blackbody radiation Ø any object in equilibrium at a fixed temperature continuously emits electromagnetic radiation (light!) with a spectrum that only depends on the temperature PHYS HON, Chapter 27 -28: Quantum Theory 4

Blackbody radiation Ø A blackbody is a theoretical substance that absorbs all incident light, and reflects nothing. Ø the light that comes from a blackbody is therefore entirely produced by it Ø the molecules and atoms in a blackbody at a temperature above absolute zero are thermally excited: they move around, vibrate, collide, etc… Ø the hotter the object, the more the particles inside it move Ø if the object contains charged particles (i. e. , electrons and protons), the motion will cause them to radiate electromagnetic energy … this is why blackbodies emit light PHYS HON, Chapter 27 -28: Quantum Theory 5

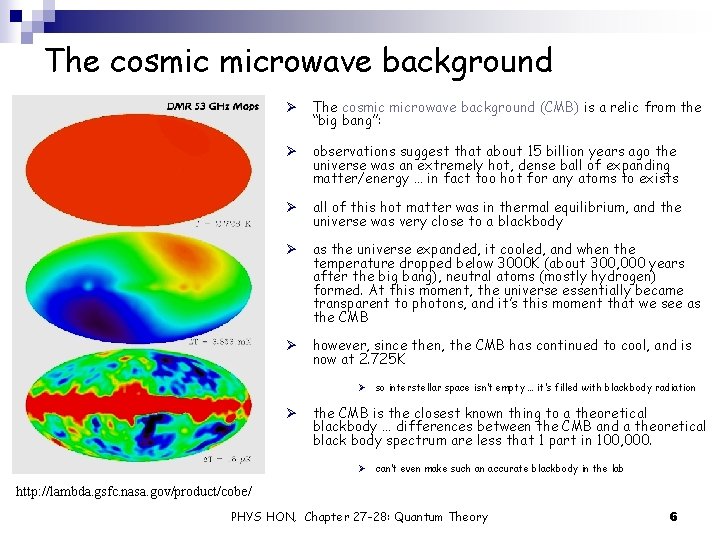
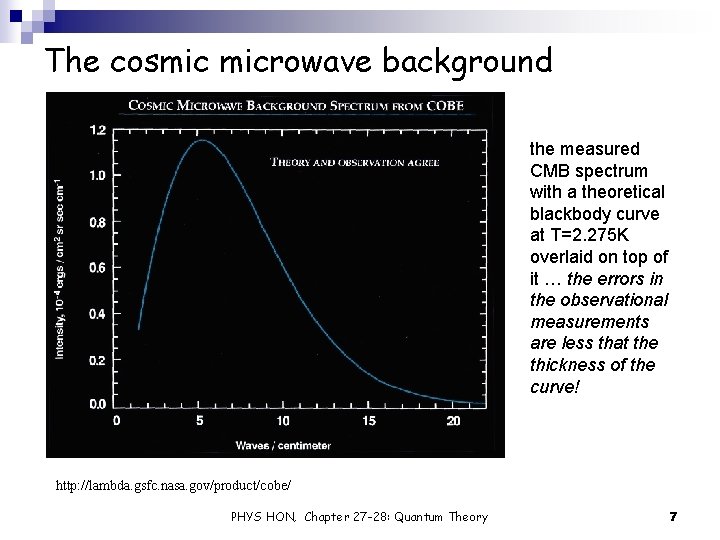
The cosmic microwave background Ø The cosmic microwave background (CMB) is a relic from the “big bang”: Ø observations suggest that about 15 billion years ago the universe was an extremely hot, dense ball of expanding matter/energy … in fact too hot for any atoms to exists Ø all of this hot matter was in thermal equilibrium, and the universe was very close to a blackbody Ø as the universe expanded, it cooled, and when the temperature dropped below 3000 K (about 300, 000 years after the big bang), neutral atoms (mostly hydrogen) formed. At this moment, the universe essentially became transparent to photons, and it’s this moment that we see as the CMB Ø however, since then, the CMB has continued to cool, and is now at 2. 725 K Ø so interstellar space isn’t empty … it’s filled with blackbody radiation Ø the CMB is the closest known thing to a theoretical blackbody … differences between the CMB and a theoretical black body spectrum are less that 1 part in 100, 000. Ø can’t even make such an accurate blackbody in the lab http: //lambda. gsfc. nasa. gov/product/cobe/ PHYS HON, Chapter 27 -28: Quantum Theory 6

The cosmic microwave background the measured CMB spectrum with a theoretical blackbody curve at T=2. 275 K overlaid on top of it … the errors in the observational measurements are less that the thickness of the curve! http: //lambda. gsfc. nasa. gov/product/cobe/ PHYS HON, Chapter 27 -28: Quantum Theory 7

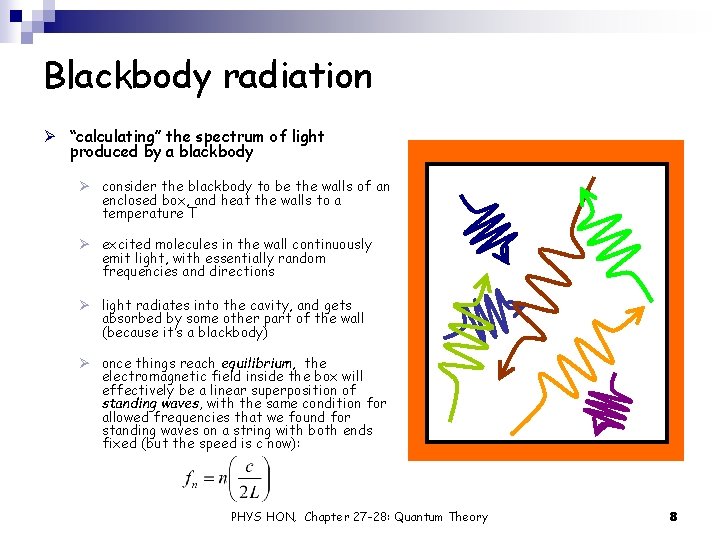
Blackbody radiation Ø “calculating” the spectrum of light produced by a blackbody Ø consider the blackbody to be the walls of an enclosed box, and heat the walls to a temperature T Ø excited molecules in the wall continuously emit light, with essentially random frequencies and directions Ø light radiates into the cavity, and gets absorbed by some other part of the wall (because it’s a blackbody) Ø once things reach equilibrium, the electromagnetic field inside the box will effectively be a linear superposition of standing waves, with the same condition for allowed frequencies that we found for standing waves on a string with both ends fixed (but the speed is c now): PHYS HON, Chapter 27 -28: Quantum Theory 8

Blackbody radiation Ø The spectrum of standing waves inside the box is the intensity (energy density) of each wavelength of light that exists inside the box Ø to calculate this, note: Ø waves carry energy: classically (i. e. pre-quantum), the energy of a wave is proportional to the square of its amplitude Ø in other words, all waves of the same amplitude have the same energy, regardless of their frequency Ø temperature is a measure of energy: at a given temperature, the walls of the blackbody have a fixed about of thermal energy. Thus (by conservation of energy) there is a fixed amount of energy that can be radiated into the inside of the box. Ø because of the random nature of the emission by molecules in the wall, by the time we reach equilibrium this given amount of energy is going to be shared randomly among all the possible standing wave modes in the box PHYS HON, Chapter 27 -28: Quantum Theory 9

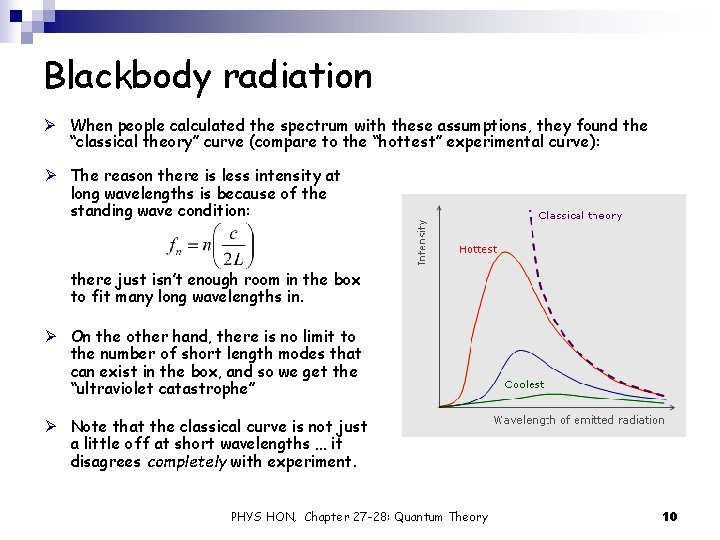
Blackbody radiation Ø When people calculated the spectrum with these assumptions, they found the “classical theory” curve (compare to the “hottest” experimental curve): Ø The reason there is less intensity at long wavelengths is because of the standing wave condition: there just isn’t enough room in the box to fit many long wavelengths in. Ø On the other hand, there is no limit to the number of short length modes that can exist in the box, and so we get the “ultraviolet catastrophe” Ø Note that the classical curve is not just a little off at short wavelengths … it disagrees completely with experiment. PHYS HON, Chapter 27 -28: Quantum Theory 10

Blackbody radiation Ø In 1900, Max Planck (1858 -1947) was able to properly calculate the spectrum of blackbody radiation following the procedure outlined on the previous slides, except he assumed that the energy of a wave is equal to an integral multiple of it’s frequency: Ø this fixes the problem with the original calculation, because now short wavelength modes (high frequency) have higher energy, and since there is a limited amount of energy that can be shared, there can’t be as many of the short wavelength modes in the spectrum Ø frequency does not have the same units as energy, so the constant of proportionality h must have SI units of joule-seconds. h today is known as Planck’s constant, and is experimentally determined to have the value: Ø At the time even Planck did not know what to make of his assumption, but it was the first hint telling of the dual particle-wave nature of light Ø each particle, or photon of light has an energy E=hf; n is therefore the number of photons. PHYS HON, Chapter 27 -28: Quantum Theory 11

Example 30. 1 Ø A red laser pointer emits light at 690 nm. The power in the laser beam is 5. 0 m. W. How many photons per second is the laser pointer emitting? PHYS HON, Chapter 27 -28: Quantum Theory 12

Classical versus Quantum regimes Ø The classical regime labels phenomena that can be explained using classical theories; the quantum regime requires ideas from modern physics Ø classical theory fails to explain phenomena in the quantum regime, but quantum theory, if it is correct, must be able to explain classical phenomena Ø In the case of the wave-particle duality and the way it relates to light, in the classical regime light has wave-like properties (as proven by Young’s double slit experiment). The laser light in the previous example is “classical” Ø this means if we measure the amplitude A of the electromagnetic waves of the laser, we would find it be proportional to the square-root of energy output E of the laser. How does quantum theory explain that? Ø Planck’s hypothesis says : E = nhf. What we measure as the amplitude of the electromagnetic field at any moment of time is the average linear superposition of the waves from n (1. 7 x 1017 per second) identical photons. Statistical theory says that for large n, this average A will equal the square-root of n; or n=A 2. Thus E=A 2 hf for large n. i. e. consistent with the classical description of a wave! Ø So, in this example, the classical regime of laser light is the limit where the number of photons in the beam becomes very large. PHYS HON, Chapter 27 -28: Quantum Theory 13

The story so far … Ø Young’s double slit experiment seemed to provide conclusive evidence that light was wave-like Ø Planck’s calculation of the blackbody spectrum implied light was particle like Ø they’re both correct! … this is the waveparticle duality Ø Planck’s calculation also required a reinterpretation of the energy of a wave associated with a single particle : E=hf PHYS HON, Chapter 27 -28: Quantum Theory 14

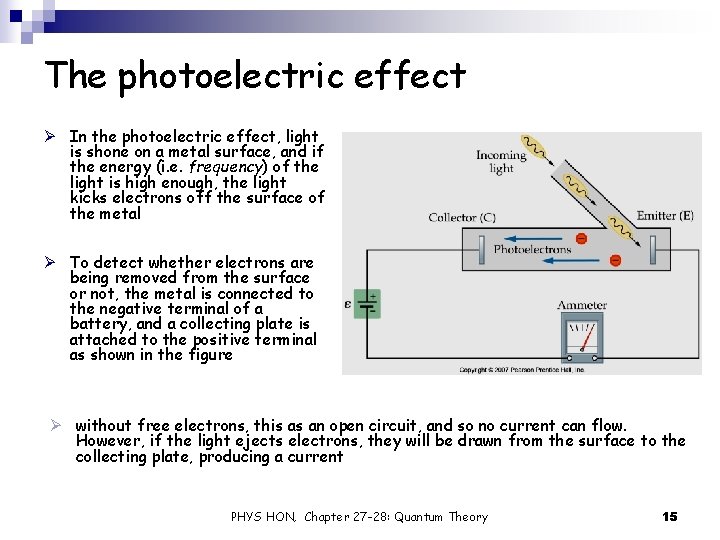
The photoelectric effect Ø In the photoelectric effect, light is shone on a metal surface, and if the energy (i. e. frequency) of the light is high enough, the light kicks electrons off the surface of the metal Ø To detect whether electrons are being removed from the surface or not, the metal is connected to the negative terminal of a battery, and a collecting plate is attached to the positive terminal as shown in the figure Ø without free electrons, this as an open circuit, and so no current can flow. However, if the light ejects electrons, they will be drawn from the surface to the collecting plate, producing a current PHYS HON, Chapter 27 -28: Quantum Theory 15

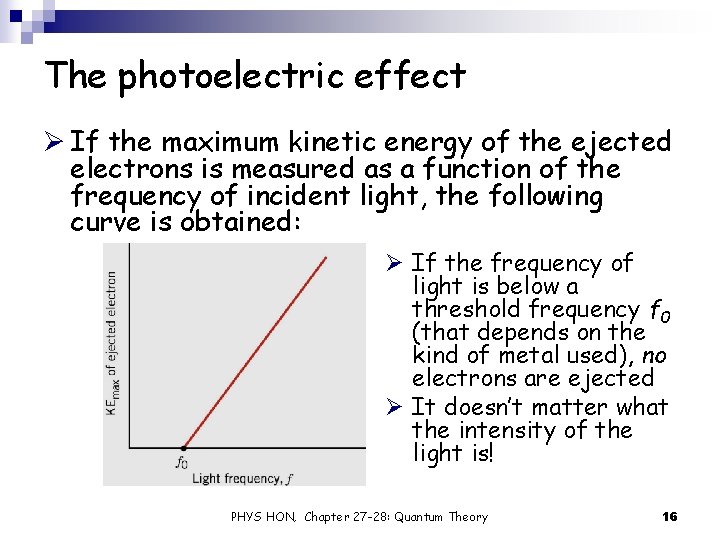
The photoelectric effect Ø If the maximum kinetic energy of the ejected electrons is measured as a function of the frequency of incident light, the following curve is obtained: Ø If the frequency of light is below a threshold frequency f 0 (that depends on the kind of metal used), no electrons are ejected Ø It doesn’t matter what the intensity of the light is! PHYS HON, Chapter 27 -28: Quantum Theory 16

The photoelectric effect Ø So what’s going on here? Using Planck’s hypothesis, Einstein in 1905 explained the photoelectric effect as follows: Ø electrons are bound to atoms in the metal Ø it takes a certain minimum amount of energy, that Einstein labeled the work function W 0, to remove the electron from the atom Ø the work function depends on the kind of metal, i. e. how electrons are bonded to the protons in the atoms of the metal Ø if a photon of light hits an atom, it can be absorbed by the atom. If the energy E=hf of the photon is high enough, i. e. greater than or equal to W 0, it will be break the bond holding the electron to the atom. PHYS HON, Chapter 27 -28: Quantum Theory 17

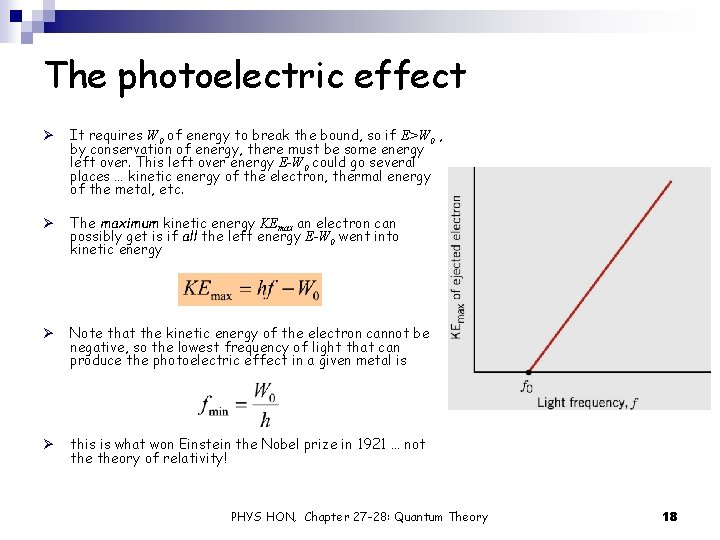
The photoelectric effect Ø It requires W 0 of energy to break the bound, so if E>W 0 , by conservation of energy, there must be some energy left over. This left over energy E-W 0 could go several places … kinetic energy of the electron, thermal energy of the metal, etc. Ø The maximum kinetic energy KEmax an electron can possibly get is if all the left energy E-W 0 went into kinetic energy Ø Note that the kinetic energy of the electron cannot be negative, so the lowest frequency of light that can produce the photoelectric effect in a given metal is Ø this is what won Einstein the Nobel prize in 1921 … not theory of relativity! PHYS HON, Chapter 27 -28: Quantum Theory 18

The photoelectric effect Ø Why could classical theory not explain the photoelectric effect? Ø because classically the energy of light is not related to the frequency of the light wave, so what should have turned the photoelectric effect “on” was merely having light with sufficient intensity Ø in the quantum explanation, the intensity of the light is related to the number of photons. But if an individual photon doesn’t have enough energy, it doesn’t matter how many of these you throw at the atom, they won’t be able to overcome the work function PHYS HON, Chapter 27 -28: Quantum Theory 19

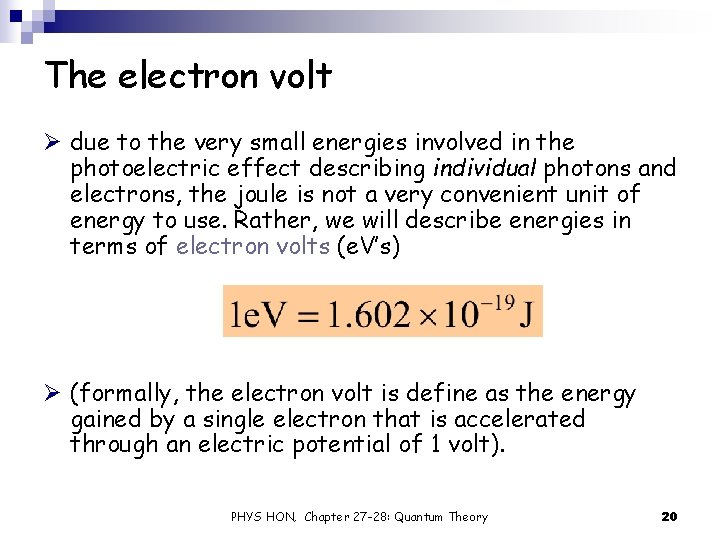
The electron volt Ø due to the very small energies involved in the photoelectric effect describing individual photons and electrons, the joule is not a very convenient unit of energy to use. Rather, we will describe energies in terms of electron volts (e. V’s) Ø (formally, the electron volt is define as the energy gained by a single electron that is accelerated through an electric potential of 1 volt). PHYS HON, Chapter 27 -28: Quantum Theory 20

Example 30. 2 Ø What is the energy of a single photon of blue (420 nm) light in a) joules and b) electron volts? PHYS HON, Chapter 27 -28: Quantum Theory 21

Example 30. 3 Ø A silver surface has a work function of W 0=4. 73 e. V. a) What is the maximum wavelength that light shining on the surface can have and still eject electrons from the surface? b) suppose light of half this wavelength lands on the surface, what is the maximum velocity that ejected electrons will have? (me=9. 11 x 10 -31 kg) PHYS HON, Chapter 27 -28: Quantum Theory 22

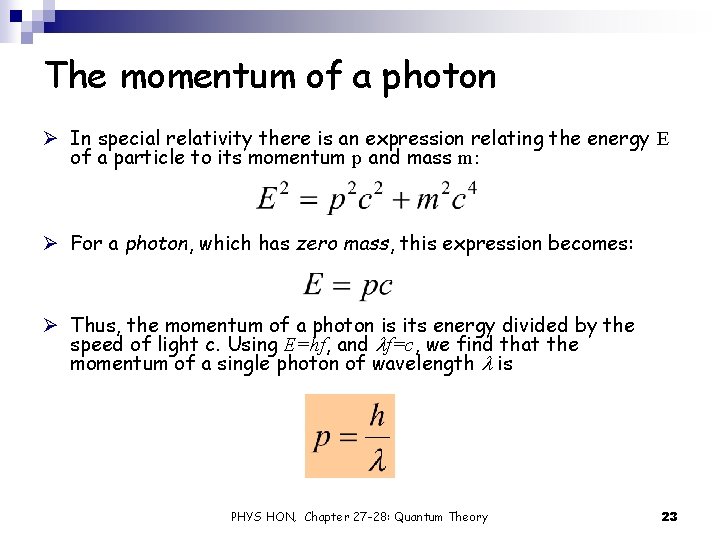
The momentum of a photon Ø In special relativity there is an expression relating the energy E of a particle to its momentum p and mass m: Ø For a photon, which has zero mass, this expression becomes: Ø Thus, the momentum of a photon is its energy divided by the speed of light c. Using E=hf, and lf=c, we find that the momentum of a single photon of wavelength l is PHYS HON, Chapter 27 -28: Quantum Theory 23

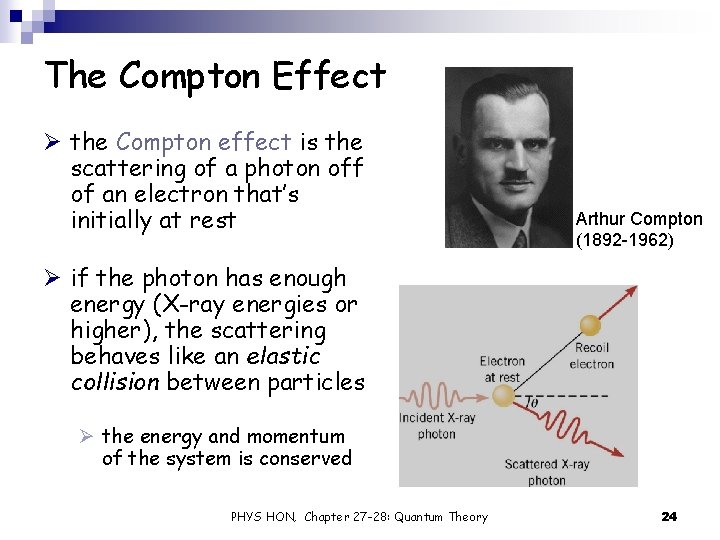
The Compton Effect Ø the Compton effect is the scattering of a photon off of an electron that’s initially at rest Arthur Compton (1892 -1962) Ø if the photon has enough energy (X-ray energies or higher), the scattering behaves like an elastic collision between particles Ø the energy and momentum of the system is conserved PHYS HON, Chapter 27 -28: Quantum Theory 24

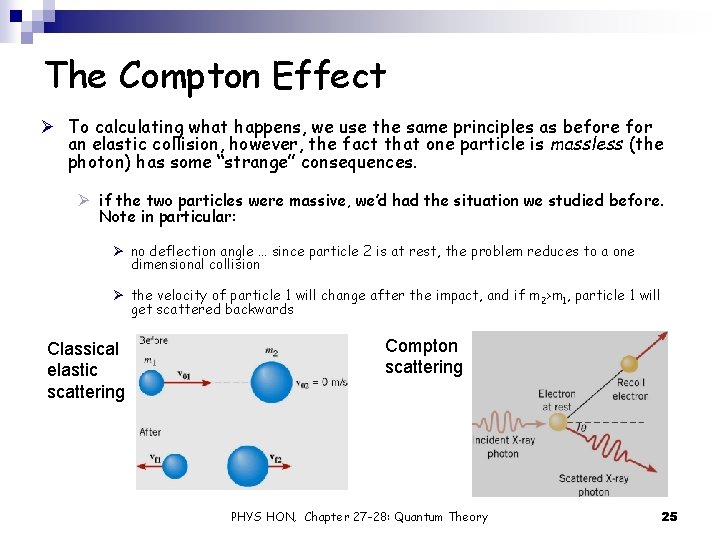
The Compton Effect Ø To calculating what happens, we use the same principles as before for an elastic collision, however, the fact that one particle is massless (the photon) has some “strange” consequences. Ø if the two particles were massive, we’d had the situation we studied before. Note in particular: Ø no deflection angle … since particle 2 is at rest, the problem reduces to a one dimensional collision Ø the velocity of particle 1 will change after the impact, and if m 2>m 1, particle 1 will get scattered backwards Classical elastic scattering Compton scattering PHYS HON, Chapter 27 -28: Quantum Theory 25

The Compton Effect Ø Since the photon is massless, it always moves at the speed of light. Ø the photon does loose momentum and energy during the collision (giving it to the electron), consequently its wavelength decreases Ø the “reason” there is a deflection angle, is that otherwise it would be impossible for the system to conserve both energy and linear momentum PHYS HON, Chapter 27 -28: Quantum Theory 26

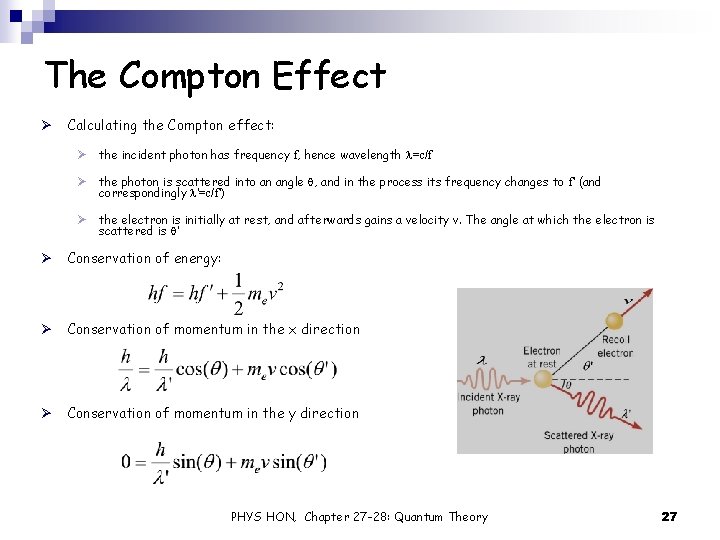
The Compton Effect Ø Calculating the Compton effect: Ø the incident photon has frequency f, hence wavelength l=c/f Ø the photon is scattered into an angle q, and in the process its frequency changes to f’ (and correspondingly l’=c/f’) Ø the electron is initially at rest, and afterwards gains a velocity v. The angle at which the electron is scattered is q’ Ø Conservation of energy: Ø Conservation of momentum in the x direction Ø Conservation of momentum in the y direction PHYS HON, Chapter 27 -28: Quantum Theory 27

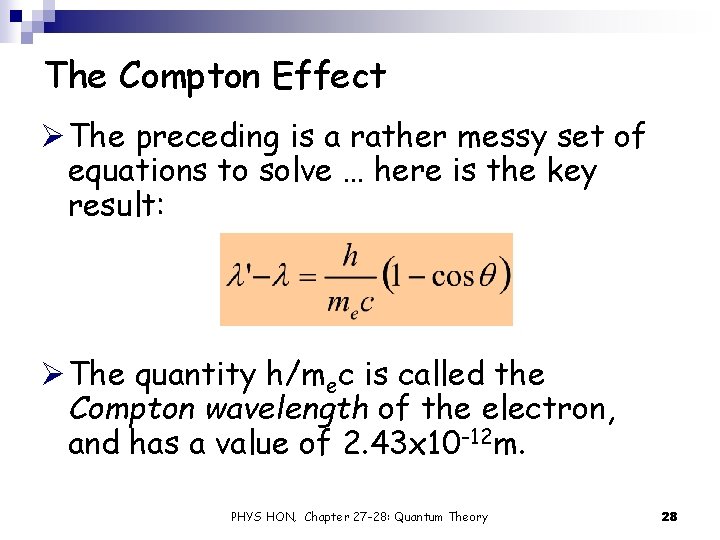
The Compton Effect Ø The preceding is a rather messy set of equations to solve … here is the key result: Ø The quantity h/mec is called the Compton wavelength of the electron, and has a value of 2. 43 x 10 -12 m. PHYS HON, Chapter 27 -28: Quantum Theory 28

Example 30. 4 Ø In a Compton scattering experiment, the incident x-rays have a wavelength of 0. 2685 nm, while the scattered x-rays have a wavelength of 0. 2703 nm. Through what angle are the x-rays scattered? PHYS HON, Chapter 27 -28: Quantum Theory 29

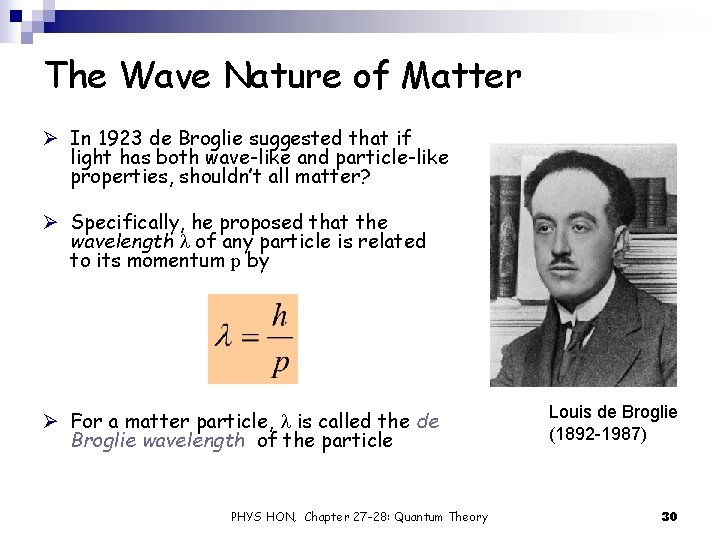
The Wave Nature of Matter Ø In 1923 de Broglie suggested that if light has both wave-like and particle-like properties, shouldn’t all matter? Ø Specifically, he proposed that the wavelength l of any particle is related to its momentum p by Ø For a matter particle, l is called the de Broglie wavelength of the particle PHYS HON, Chapter 27 -28: Quantum Theory Louis de Broglie (1892 -1987) 30

Example 30. 5 Ø a) What is the de Broglie wavelength of an electron moving at 1. 0 x 106 m/s (similar to the velocity of ejected electrons from the photoelectric effect in example 30. 3) ? If we were trying to test the wave nature of the electron, and shone a beam of electrons (with v= 1. 0 x 106 m/s) at a double slit, what would the separation of the slits need to be to produce an interference pattern where the first bright fringe is a distance of 1. 0 mm from the central fringe, when viewed a distance of 3. 0 m from the slit? PHYS HON, Chapter 27 -28: Quantum Theory 31

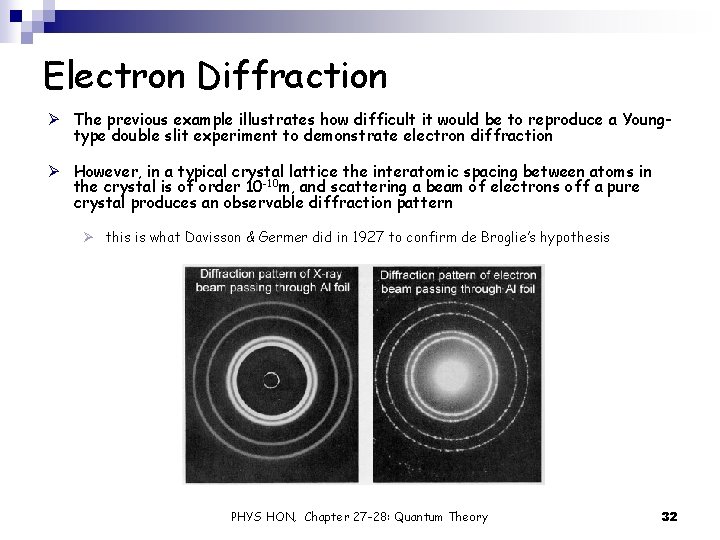
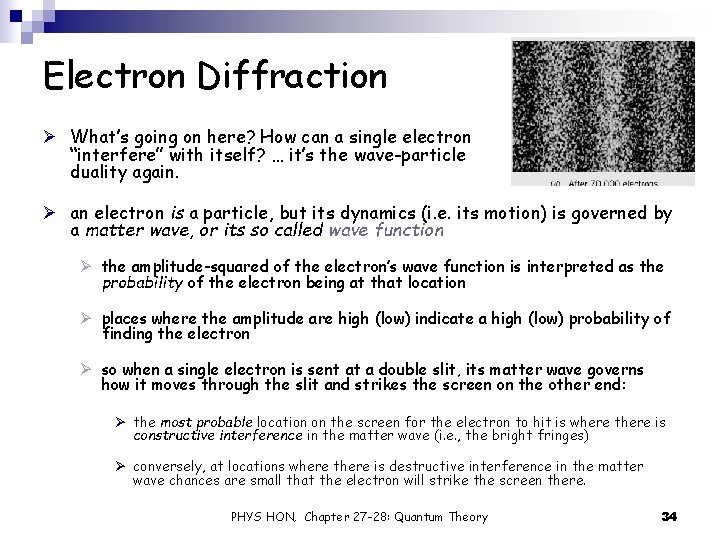
Electron Diffraction Ø The previous example illustrates how difficult it would be to reproduce a Youngtype double slit experiment to demonstrate electron diffraction Ø However, in a typical crystal lattice the interatomic spacing between atoms in the crystal is of order 10 -10 m, and scattering a beam of electrons off a pure crystal produces an observable diffraction pattern Ø this is what Davisson & Germer did in 1927 to confirm de Broglie’s hypothesis PHYS HON, Chapter 27 -28: Quantum Theory 32

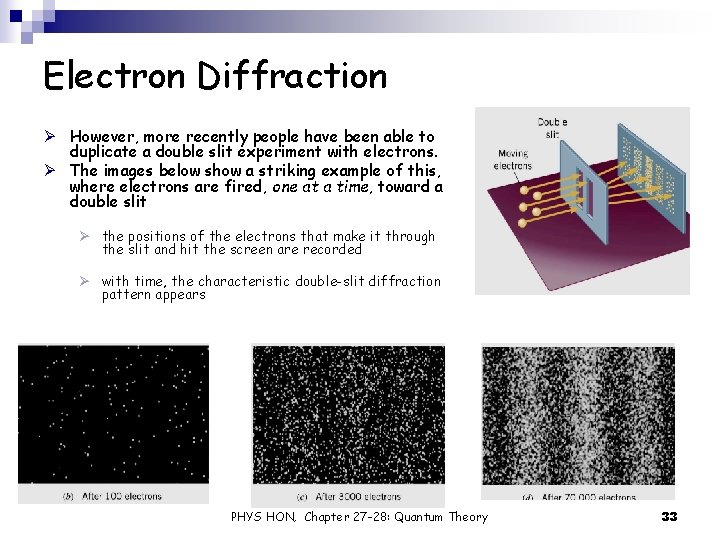
Electron Diffraction Ø However, more recently people have been able to duplicate a double slit experiment with electrons. Ø The images below show a striking example of this, where electrons are fired, one at a time, toward a double slit Ø the positions of the electrons that make it through the slit and hit the screen are recorded Ø with time, the characteristic double-slit diffraction pattern appears PHYS HON, Chapter 27 -28: Quantum Theory 33

Electron Diffraction Ø What’s going on here? How can a single electron “interfere” with itself? … it’s the wave-particle duality again. Ø an electron is a particle, but its dynamics (i. e. its motion) is governed by a matter wave, or its so called wave function Ø the amplitude-squared of the electron’s wave function is interpreted as the probability of the electron being at that location Ø places where the amplitude are high (low) indicate a high (low) probability of finding the electron Ø so when a single electron is sent at a double slit, its matter wave governs how it moves through the slit and strikes the screen on the other end: Ø the most probable location on the screen for the electron to hit is where there is constructive interference in the matter wave (i. e. , the bright fringes) Ø conversely, at locations where there is destructive interference in the matter wave chances are small that the electron will strike the screen there. PHYS HON, Chapter 27 -28: Quantum Theory 34

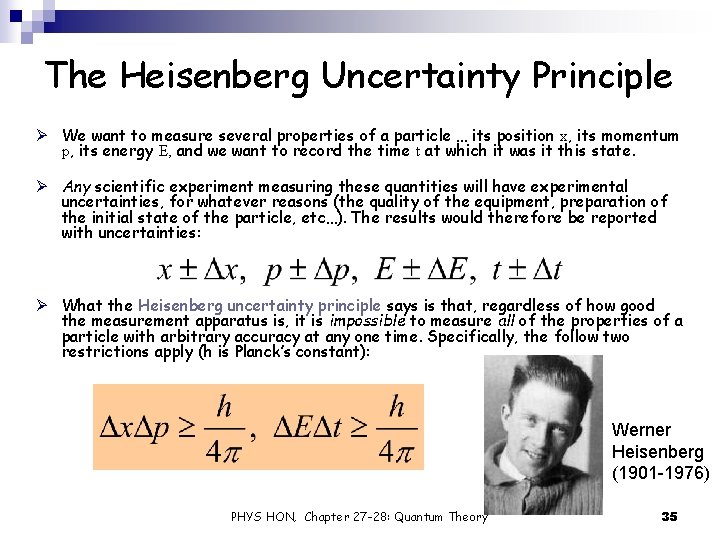
The Heisenberg Uncertainty Principle Ø We want to measure several properties of a particle … its position x, its momentum p, its energy E, and we want to record the time t at which it was it this state. Ø Any scientific experiment measuring these quantities will have experimental uncertainties, for whatever reasons (the quality of the equipment, preparation of the initial state of the particle, etc…). The results would therefore be reported with uncertainties: Ø What the Heisenberg uncertainty principle says is that, regardless of how good the measurement apparatus is, it is impossible to measure all of the properties of a particle with arbitrary accuracy at any one time. Specifically, the follow two restrictions apply (h is Planck’s constant): Werner Heisenberg (1901 -1976) PHYS HON, Chapter 27 -28: Quantum Theory 35

The Heisenberg Uncertainty Principle Ø The Heisenberg uncertainty principle is a consequence of both the wave-particle duality, and the probabilistic interpretation of a wave function. Ø suppose we did have an electron with a definite momentum p (i. e. Dp=0). Its wave function would be a sine wave with a wavelength given by it’s de Broglie wavelength: Ø now we want to measure the position of this electron … where will it be? The probability of finding the electron at some location is proportional to the amplitude-squared of it’s wave function there … for a sine wave-wave function it could thus be anywhere! : Dx=h/(4 p. Dp)=infinity! PHYS HON, Chapter 27 -28: Quantum Theory 36

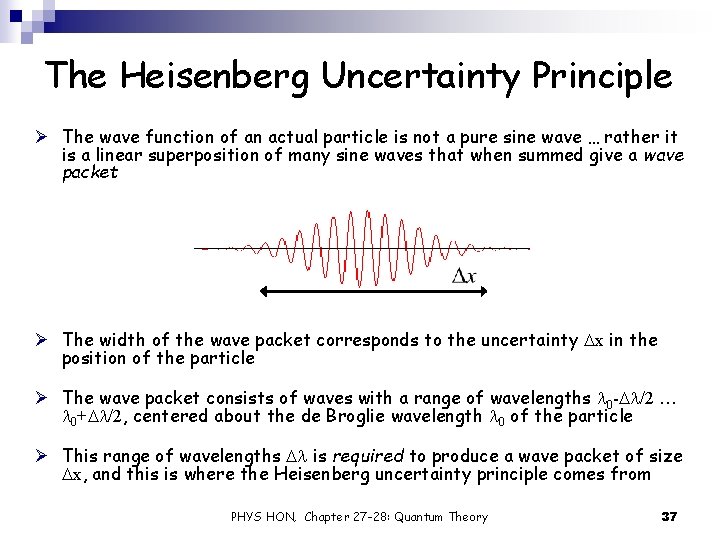
The Heisenberg Uncertainty Principle Ø The wave function of an actual particle is not a pure sine wave … rather it is a linear superposition of many sine waves that when summed give a wave packet Ø The width of the wave packet corresponds to the uncertainty Dx in the position of the particle Ø The wave packet consists of waves with a range of wavelengths l 0 -Dl/2 … l 0+Dl/2, centered about the de Broglie wavelength l 0 of the particle Ø This range of wavelengths Dl is required to produce a wave packet of size Dx, and this is where the Heisenberg uncertainty principle comes from PHYS HON, Chapter 27 -28: Quantum Theory 37

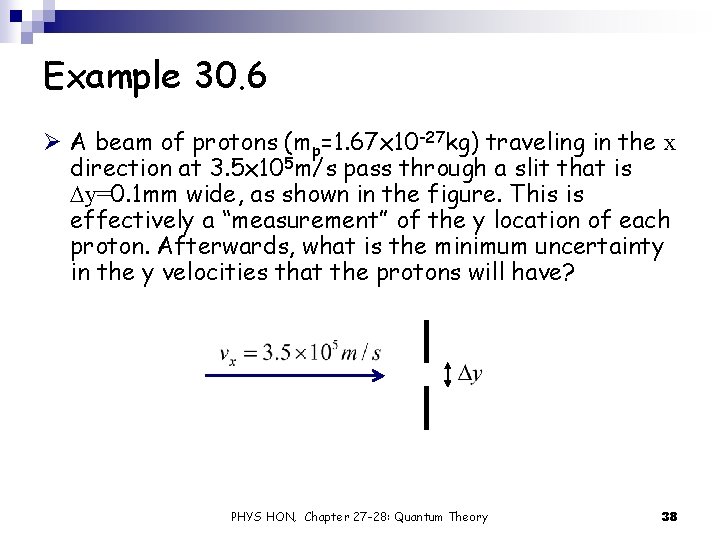
Example 30. 6 Ø A beam of protons (mp=1. 67 x 10 -27 kg) traveling in the x direction at 3. 5 x 105 m/s pass through a slit that is Dy=0. 1 mm wide, as shown in the figure. This is effectively a “measurement” of the y location of each proton. Afterwards, what is the minimum uncertainty in the y velocities that the protons will have? PHYS HON, Chapter 27 -28: Quantum Theory 38

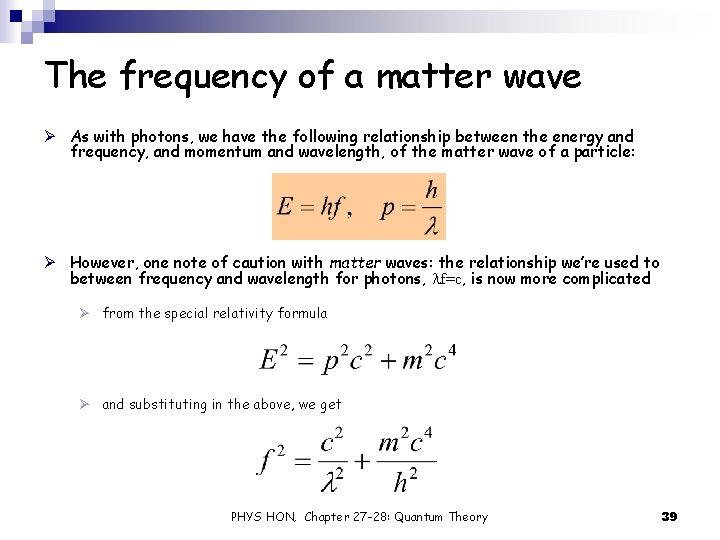
The frequency of a matter wave Ø As with photons, we have the following relationship between the energy and frequency, and momentum and wavelength, of the matter wave of a particle: Ø However, one note of caution with matter waves: the relationship we’re used to between frequency and wavelength for photons, lf=c, is now more complicated Ø from the special relativity formula Ø and substituting in the above, we get PHYS HON, Chapter 27 -28: Quantum Theory 39