Quantum Physics Waves and particles The Quantum Sun





- Slides: 20

Quantum Physics • Waves and particles • The Quantum Sun • Schrödinger’s Cat and the Quantum Code

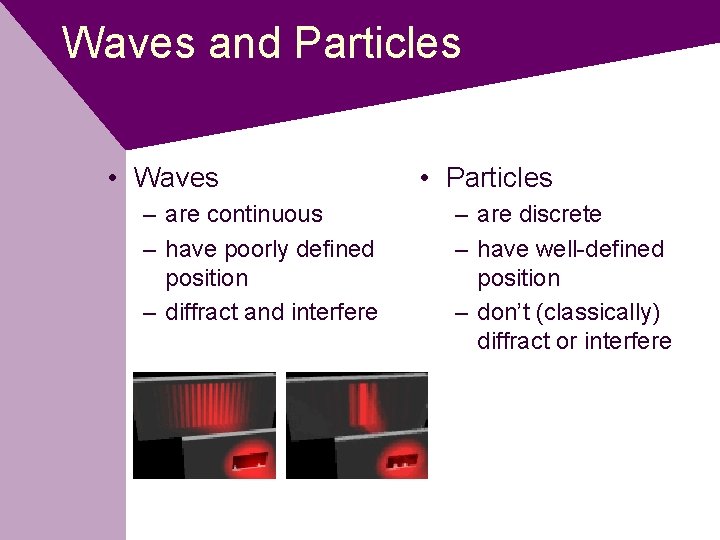
Waves and Particles • Waves – are continuous – have poorly defined position – diffract and interfere • Particles – are discrete – have well-defined position – don’t (classically) diffract or interfere

Light is a wave • Thomas Young (1773– 1829) – light undergoes diffraction and interference (Young’s slits) – (also: theory of colour vision, compressibility of materials (Young’s modulus), near-decipherment of Egyptian hieroglyphs—clever chap…) • James Clerk Maxwell (1831– 79) – light as an electromagnetic wave – (and colour photography, thermodynamics, Saturn’s rings—incredibly clever chap…)

Light is particles • Blackbody spectrum – light behaves as if it came in packets of energy hf (Max Planck) • Photoelectric effect – light does come in packets of energy hf (Einstein) – used to measure h by Millikan in 1916

Photoelectric effect • Light causes emission of electrons from metals – energy of electrons depends on frequency of light, KE = hf – w – rate of emission (current) depends on intensity of light – this is inexplicable if light is a continuous wave, but simple to understand if it is composed of particles (photons) of energy hf

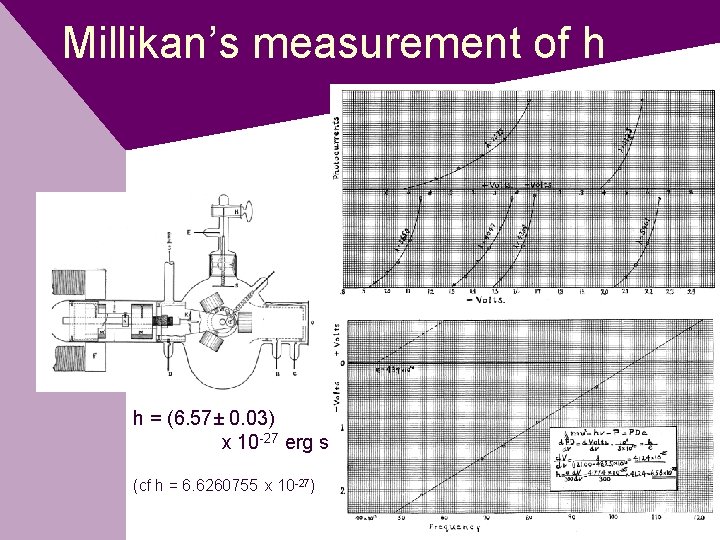
Millikan’s measurement of h h = (6. 57± 0. 03) x 10 -27 erg s (cf h = 6. 6260755 x 10 -27)

Electrons are particles • JJ Thomson (1856– 1940) – “cathode rays” have well-defined e/m (1897) • RA Millikan – measured e using oil drop experiment (1909)

Electrons are waves • GP Thomson (1892– 1975) – electrons undergo diffraction – they behave as waves with wavelength h/p • JJ Thomson won the Nobel Prize for Physics in 1906 for demonstrating that the electron is a particle. • GP Thomson (son of JJ) won it in 1937 for demonstrating that the electron is a wave. • And they were both right!

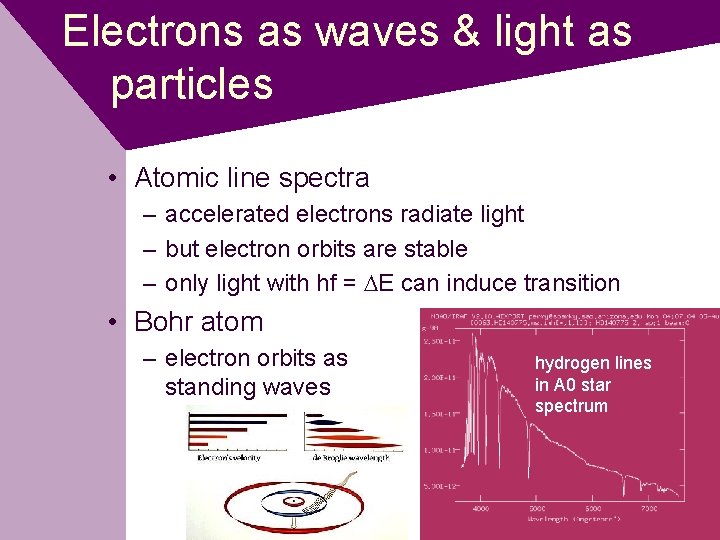
Electrons as waves & light as particles • Atomic line spectra – accelerated electrons radiate light – but electron orbits are stable – only light with hf = DE can induce transition • Bohr atom – electron orbits as standing waves hydrogen lines in A 0 star spectrum

The Uncertainty Principle • Consider measuring position of a particle – – hit it with photon of wavelength l position determined to precision Dx ~ ±l/2 but have transferred momentum Dp ~ h/l therefore, Dx. Dp ~ h/2 (and similar relation between DE and Dt) • Impossible, even in principle, to know position and momentum of particle exactly and simultaneously

Wavefunctions • Are particles “really” waves? – particle as “wave packet” • but mathematical functions describing particles as waves sometimes give complex numbers • and confined wave packet will disperse over time • Born interpretation of “matter waves” – Intensity (square of amplitude) of wave at (x, t) represents probability of finding particle there • wavefunction may be complex: probability given by Y*Y • tendency of wave packets to spread out over time represents evolution of our knowledge of the system

Postulates of Quantum Mechanics • The state of a quantum mechanical system is completely described by the wavefunction Y – wavefunction must be normalisable: ∫Y*Ydt = 1 (particle must be found somewhere!) • Observable quantities are represented by mathematical operators acting on Y • The mean value of an observable is equal to the expectation value of its corresponding operator

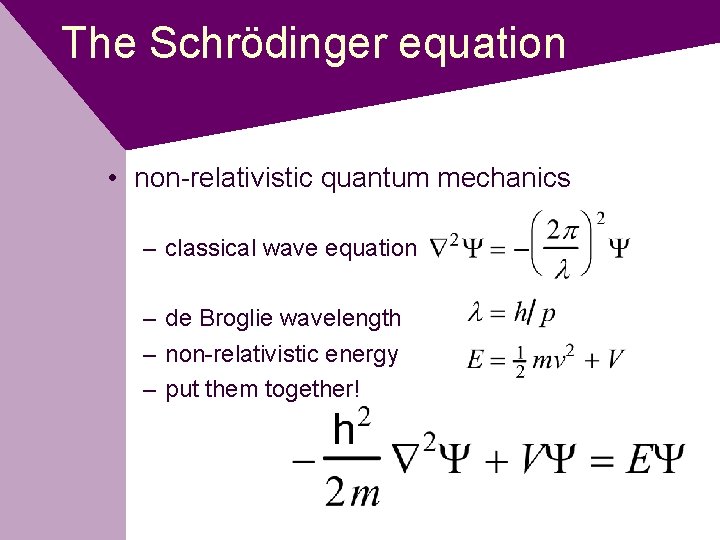
The Schrödinger equation • non-relativistic quantum mechanics – classical wave equation – de Broglie wavelength – non-relativistic energy – put them together!

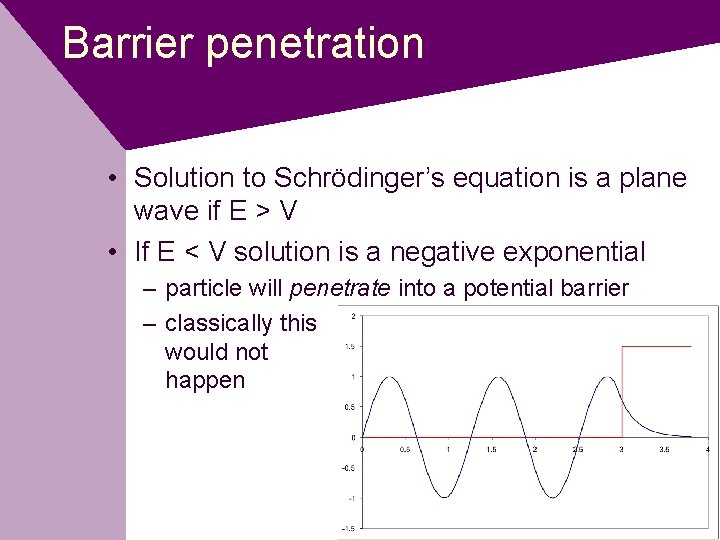
Barrier penetration • Solution to Schrödinger’s equation is a plane wave if E > V • If E < V solution is a negative exponential – particle will penetrate into a potential barrier – classically this would not happen

The Quantum Sun • Sun is powered by hydrogen fusion – protons must overcome electrostatic repulsion – thermal energy at core of Sun does not look high enough – but wavefunction penetrates into barrier (nonzero probability of finding proton inside) – tunnelling – also explains a decay

The Pauli Exclusion Principle • Identical particles are genuinely indistinguishable – if particles a and b are interchanged, either Y(a, b) = Y(b, a) or Y(a, b) = –Y(b, a) – former described bosons (force particles, mesons) latter describes fermions (quarks, leptons, baryons) – negative sign implies that two particles cannot have exactly the same quantum numbers, as Y(a, a) must be zero – Pauli Exclusion Principle

The Quantum Sun, part 2 • When the Sun runs out of hydrogen and helium to fuse, it will collapse under its own gravity • Electrons are squeezed together until all available states are full – degenerate electron gas – degeneracy pressure halts collapse – white dwarf star

Entangled states • Suppose process can have two possible outcomes – which has happened? – don’t know until we look – wavefunction of state includes both possibilities (until we look) • e. g. p 0 gg • spin 0 1+1, so g spins must be antiparallel • measuring spin of photon 1 automatically determines spin of photon 2 (even though they are separated by 2 c. Dt)

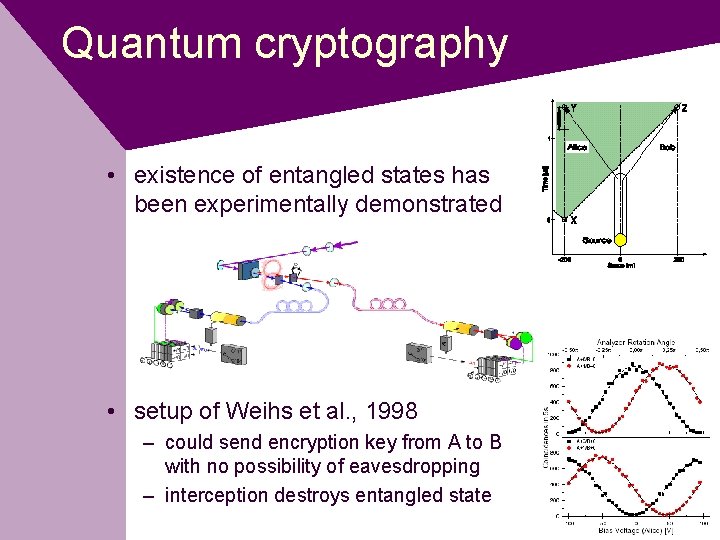
Quantum cryptography • existence of entangled states has been experimentally demonstrated • setup of Weihs et al. , 1998 – could send encryption key from A to B with no possibility of eavesdropping – interception destroys entangled state

Summary • Origin of quantum mechanics: energy of light waves comes in discrete lumps (photons) – other quantised observables: electric charge, angular momentum • Interpretation of quantum mechanics as a probabilistic view of physical processes – explains observed phenomena such as tunnelling • Possible applications include cryptography and computing – so, not as esoteric as it may appear!