Physical Science Unit 5 Test Review WarmUp Mar











































- Slides: 61

Physical Science Unit 5 Test Review

Warm-Up Mar. 12 1. How many protons does sodium (Na) have? 2. How many electrons cant he 2 nd energy level hold? 3. What is Group 18 in the periodic table called?

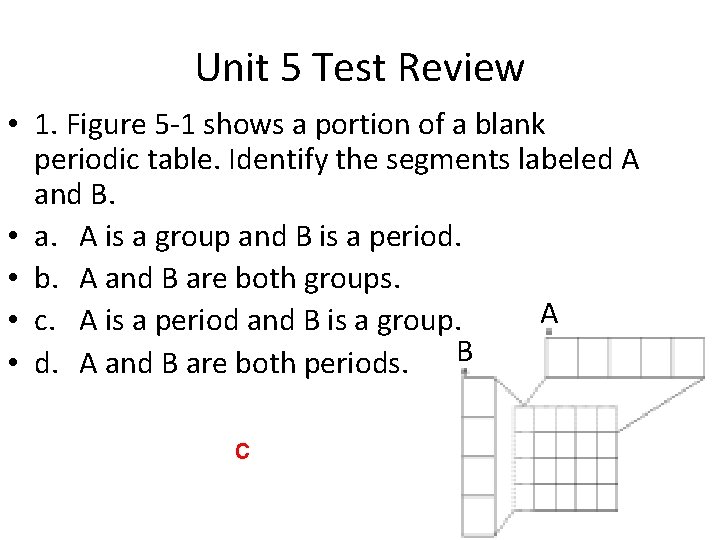
Unit 5 Test Review • 1. Figure 5 -1 shows a portion of a blank periodic table. Identify the segments labeled A and B. • a. A is a group and B is a period. • b. A and B are both groups. A • c. A is a period and B is a group. • d. A and B are both periods. B c

Unit 5 Test Review • 2. Suppose you are looking at elements in the periodic table in this order: element 23, element 24, element 25, element 26, and so on. Are you looking across a period or down a group • a. period • b. group a • c. both a & b • d. neither a nor b

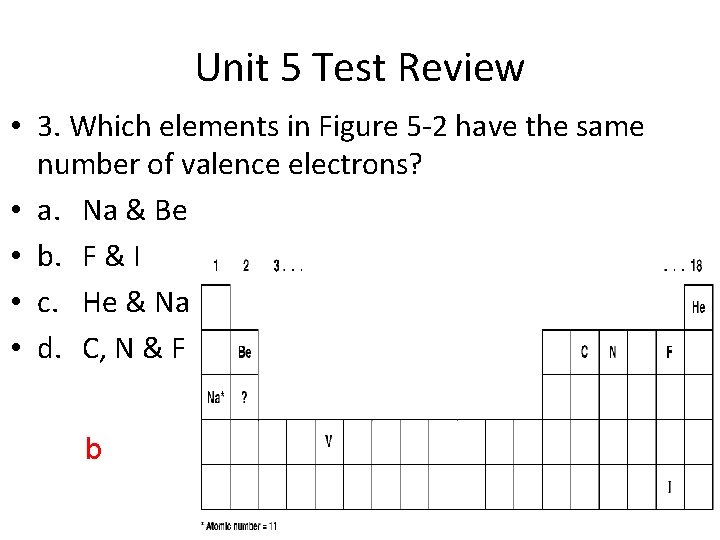
Unit 5 Test Review • 3. Which elements in Figure 5 -2 have the same number of valence electrons? • a. Na & Be • b. F & I • c. He & Na • d. C, N & F b

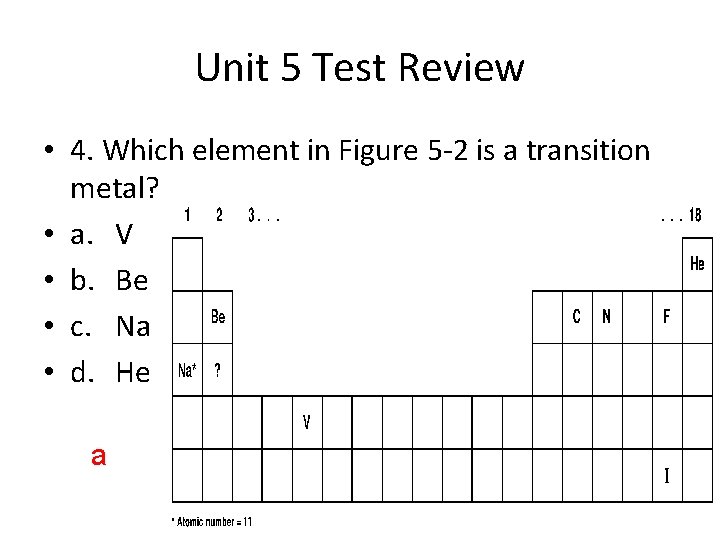
Unit 5 Test Review • 4. Which element in Figure 5 -2 is a transition metal? • a. V • b. Be • c. Na • d. He a

Unit 5 Test Review • 5. At room temperature all the elements in column 18 are • a. gas • b. liquid • c. plasma • d. solid a

Unit 5 Test Review • • • 6. Hydrogen is located in group 1 because ___ a. it is a metal b. there was no where else to put it c. it is the first element on the periodic table d. it has one valence electron d

Unit 5 Test Review • 7. The tendency of an element to react is closely related to • a. the number of valence electrons in atoms of the element. • b. attractions between its atoms. • c. its atomic mass. • d. the ratio of protons to neutrons in atoms of the element. a

Unit 5 Test Review • 8. As you move from left to right across a period, the number of valence electrons • a. stays the same. • b. increases and then decreases. • c. increases. • d. decreases. c

Unit 5 Test Review • 9. At room temperature, none of the metals are • a. malleable. • b. gases. • c. liquids. • d. soft. b

Unit 5 Test Review • 10. Which list of elements contains only metals? • a. carbon, iodine, tin • b. helium, iron, copper • c. tin, copper, cesium • d. iodine, carbon, argon c

Unit 5 Test Review • 11. Metal, Metalloid, or Nonmetal? Neon nonmetal

Unit 5 Test Review • 12. Metal, Metalloid, or Nonmetal? Gallium metal

Unit 5 Test Review • 13. What is the name of the group that Argon (Ar) is in? Noble gases

Unit 5 Test Review • 14. What is the name of the group that Iodine (I) is in? halogens

Unit 5 Test Review • 15. What is the name of the group that Calcium (Ca) is in? Alkaline earth metals

Unit 5 Test Review • 16. What is the name of the group that Chromium (Cr) is in? Transition metals

Unit 5 Test Review • 17. Which of the following is not a characteristic of the elements fluorine and chlorine? • A. brittle • B. malleable • C. gas at room temperature • D. good insulator b

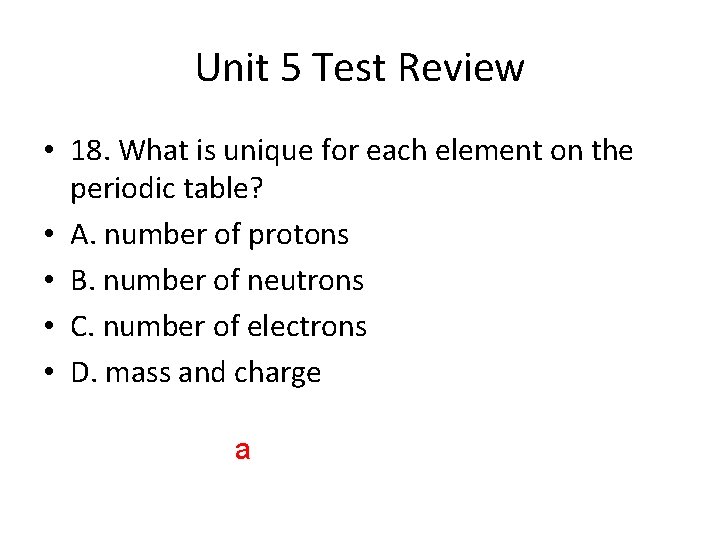
Unit 5 Test Review • 18. What is unique for each element on the periodic table? • A. number of protons • B. number of neutrons • C. number of electrons • D. mass and charge a

Unit 5 Test Review • 19. Where on the periodic table are most of the nonmetals? • A. on the left • B. on the bottom • C. on the right • D. evenly distributed c

Unit 5 Test Review • • • 20. What period number is Barium in? A. 1 B. 2 C. 4 D. 5 E. 6 e

Unit 5 Test Review • 21. How many valence electrons does Iodine have? • A. 53 • B. 127 • C. 7 • D. 17 c

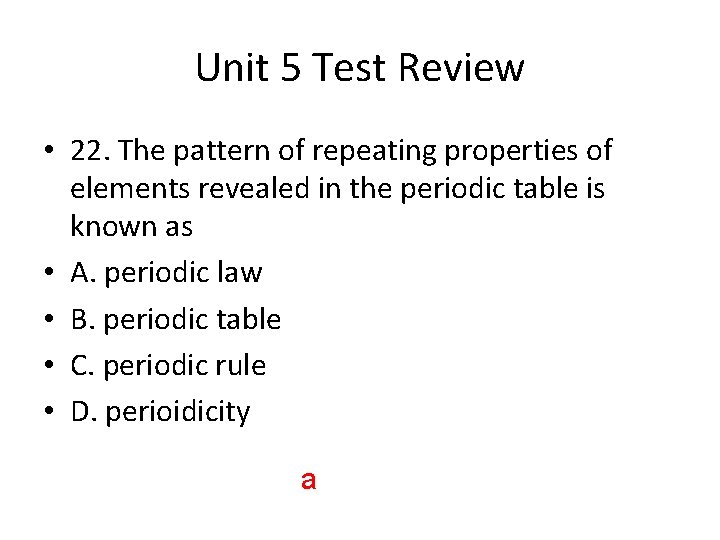
Unit 5 Test Review • 22. The pattern of repeating properties of elements revealed in the periodic table is known as • A. periodic law • B. periodic table • C. periodic rule • D. perioidicity a

Unit 5 Test Review • 23. True or False. Mendeleev’s periodic table was arranged based on atomic number, whereas the modern periodic table is arranged based on atomic mass. False

Unit 5 Test Review • • • 24. The unit for atomic mass is A. gram B. amu C. pound D. none of the above B

Unit 5 Test Review • • • 25. Group 17 of the periodic table contains the A. most reactive nonmetals. B. most reactive metals. C. least reactive nonmetals. D. least reactive metals. A

Unit 5 Test Review • 26. Atoms of the most reactive elements tend to have • A. one or seven valence electrons. • B. eight valence electrons. • C. four or five valence electrons. • D. no valence electrons. A

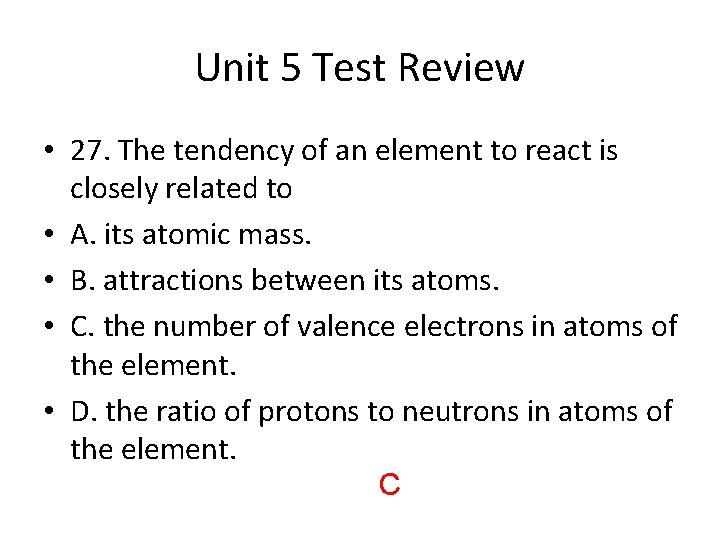
Unit 5 Test Review • 27. The tendency of an element to react is closely related to • A. its atomic mass. • B. attractions between its atoms. • C. the number of valence electrons in atoms of the element. • D. the ratio of protons to neutrons in atoms of the element. C

Unit 5 Test Review • • • 28. Which halogen is most likely to react? A. Br B. F C. I D. Cl B

Unit 5 Test Review • 29. Which of the following Group 1 elements is the most reactive? • A. Cs • B. Li • C. K • D. Na A

Unit 5 Test Review • 30. Fluorine forms a binary compound with lithium. What is the name of this compound? – A. lithium fluorine – B. lithium fluoride – C. fluorine lithium – D. fluorine lithide B

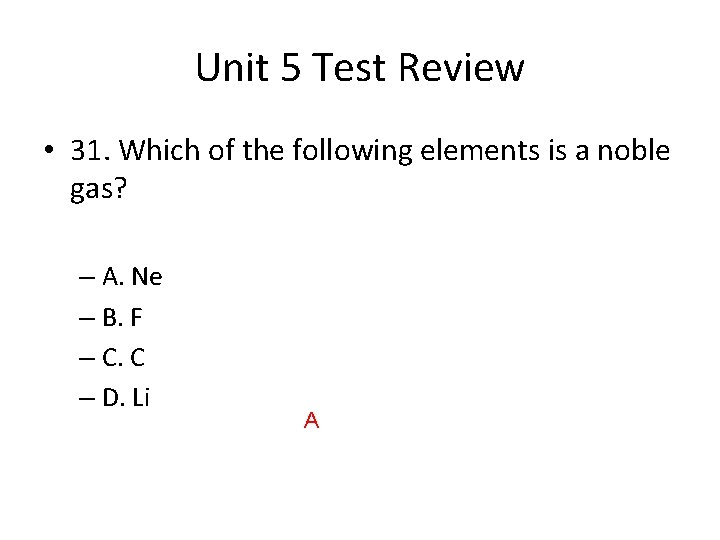
Unit 5 Test Review • 31. Which of the following elements is a noble gas? – A. Ne – B. F – C. C – D. Li A

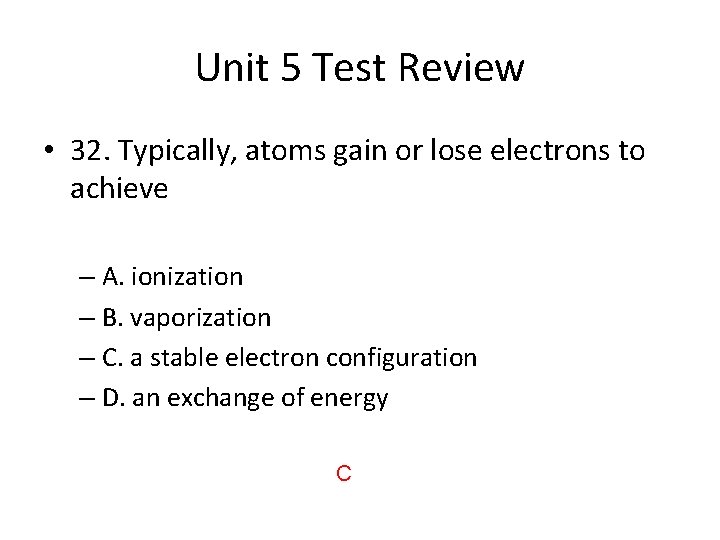
Unit 5 Test Review • 32. Typically, atoms gain or lose electrons to achieve – A. ionization – B. vaporization – C. a stable electron configuration – D. an exchange of energy C

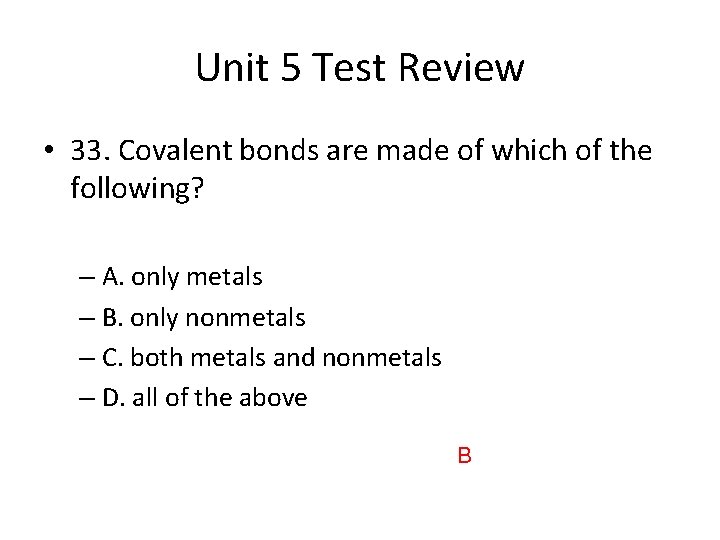
Unit 5 Test Review • 33. Covalent bonds are made of which of the following? – A. only metals – B. only nonmetals – C. both metals and nonmetals – D. all of the above B

Unit 5 Test Review • 34. In the name carbon dioxide, the prefix of the second word indicates that a molecule of carbon dioxide contains – A. a polyatomic ion – B. two oxygen atoms – C. two carbon atoms – D. an ionic bond B

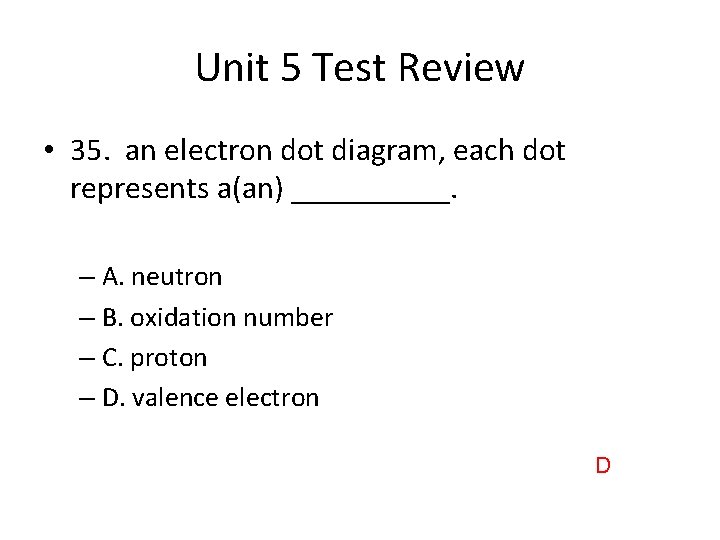
Unit 5 Test Review • 35. an electron dot diagram, each dot represents a(an) _____. – A. neutron – B. oxidation number – C. proton – D. valence electron D

Unit 5 Test Review • 36. Which of the following groups contain three elements with stable electron configurations? – A. argon, neon, barium – B. xenon, neon, boron – C. lithium, krypton, argon – D. helium, xenon, neon D

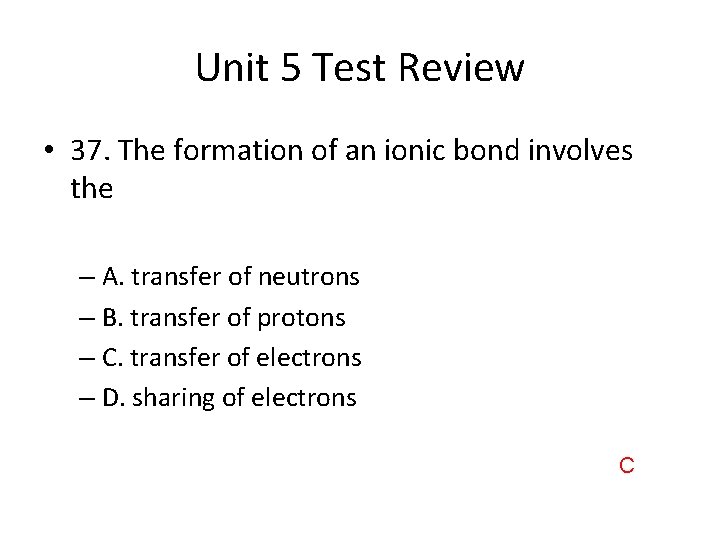
Unit 5 Test Review • 37. The formation of an ionic bond involves the – A. transfer of neutrons – B. transfer of protons – C. transfer of electrons – D. sharing of electrons C

Unit 5 Test Review • 38. The compound whose formula is SO 3 is called – A. sulfur trioxide – B. sulfur oxygen – C. monosulfur trioxide – D. sulfur oxide A

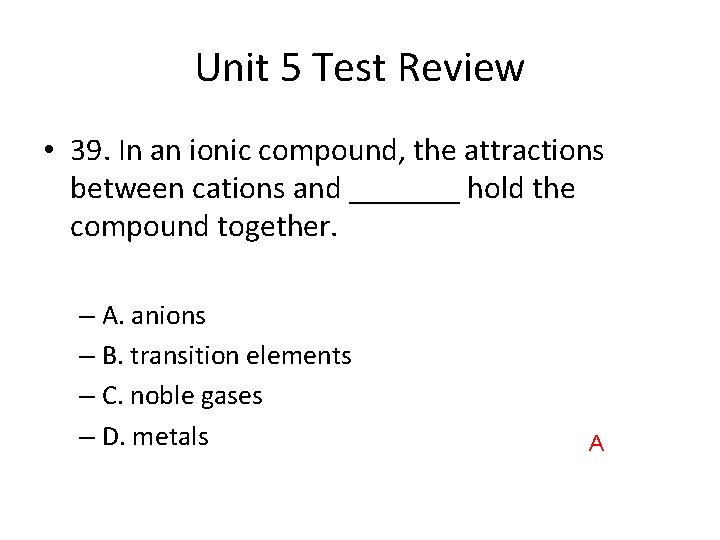
Unit 5 Test Review • 39. In an ionic compound, the attractions between cations and _______ hold the compound together. – A. anions – B. transition elements – C. noble gases – D. metals A

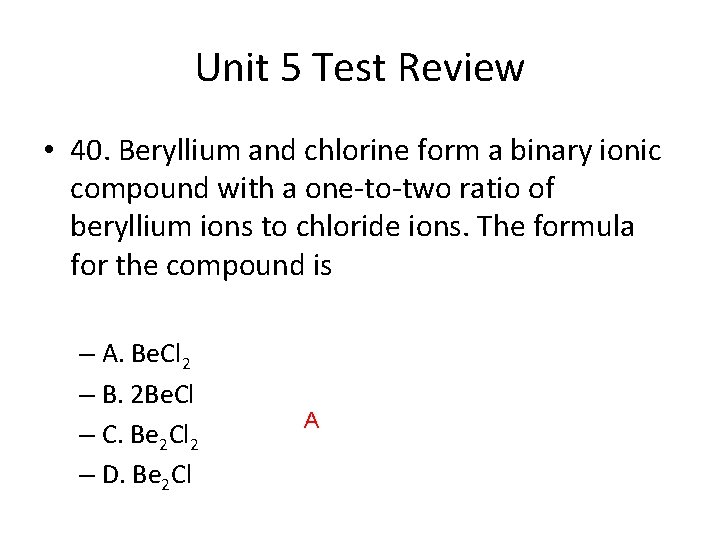
Unit 5 Test Review • 40. Beryllium and chlorine form a binary ionic compound with a one-to-two ratio of beryllium ions to chloride ions. The formula for the compound is – A. Be. Cl 2 – B. 2 Be. Cl – C. Be 2 Cl 2 – D. Be 2 Cl A

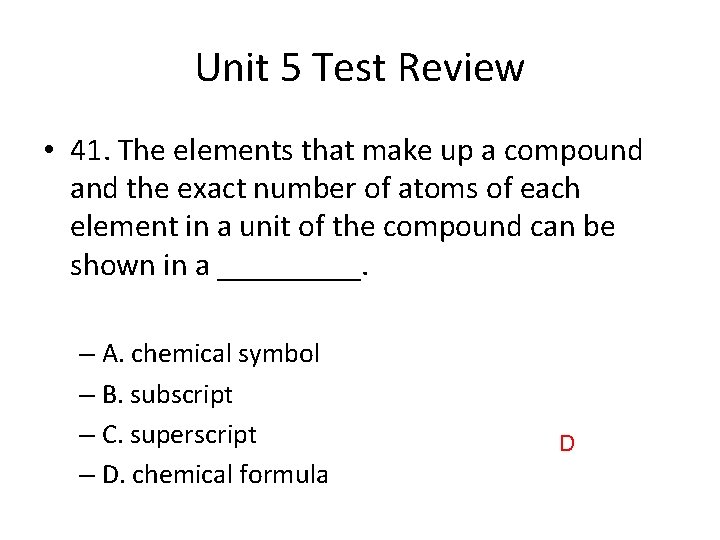
Unit 5 Test Review • 41. The elements that make up a compound and the exact number of atoms of each element in a unit of the compound can be shown in a _____. – A. chemical symbol – B. subscript – C. superscript – D. chemical formula D

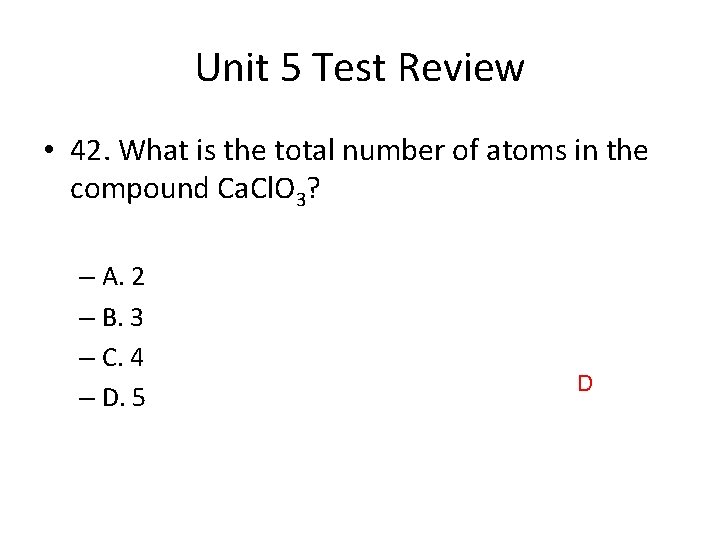
Unit 5 Test Review • 42. What is the total number of atoms in the compound Ca. Cl. O 3? – A. 2 – B. 3 – C. 4 – D. 5 D

Unit 5 Test Review • 43. What is the name of the compound with the formula Na. Cl? – A. chlorine sodium – B. sodium chlorate – C. sodium chloride – D. sodium dichloride C

Unit 5 Test Review • 44. What is the correct formula for magnesium oxide? – A. Mg. O – B. Mg. O 2 – C. Mg 2 O 2 – D. Mg 2 O A

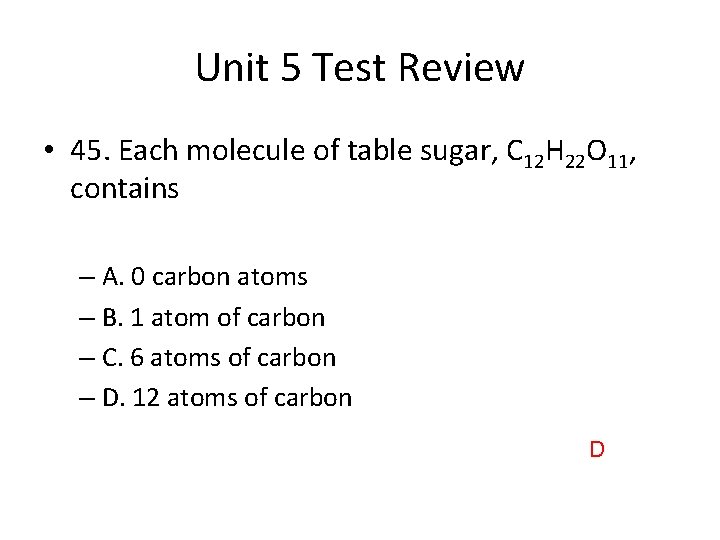
Unit 5 Test Review • 45. Each molecule of table sugar, C 12 H 22 O 11, contains – A. 0 carbon atoms – B. 1 atom of carbon – C. 6 atoms of carbon – D. 12 atoms of carbon D

Unit 5 Test Review • 46. An anion has a ______ charge. – A. positive – B. negative – C. neutral – D. both positive and negative B

Unit 5 Test Review • 47. The name dinitrogen tetroxide tells you that this compound contains – A. two nitrogen atoms and two oxygen atoms – B. four nitrogen atoms and two oxygen atoms – C. two nitrogen atoms and four oxygen atoms – D. four nitrogen atoms and four oxygen atoms C

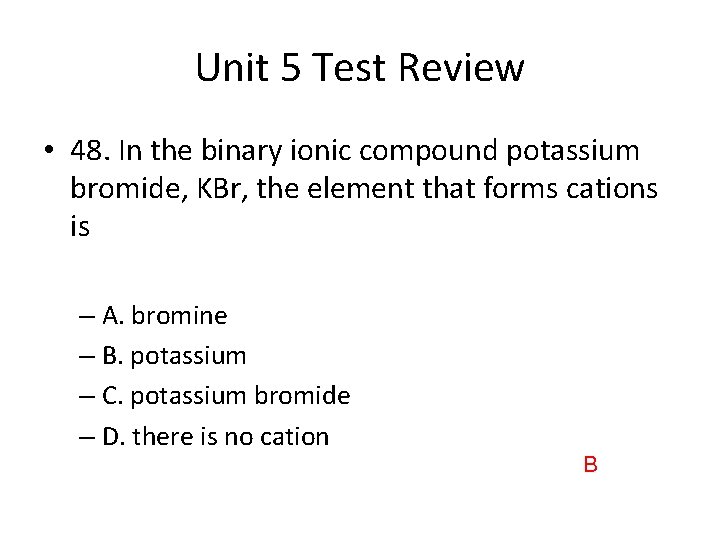
Unit 5 Test Review • 48. In the binary ionic compound potassium bromide, KBr, the element that forms cations is – A. bromine – B. potassium – C. potassium bromide – D. there is no cation B

Unit 5 Test Review • 49. True or False • Particles formed from the covalent bonding of atoms are called ions. False

Unit 5 Test Review • 50. True or False • When an atom gains or loses electrons, the charged particle that results is called a molecule. False

Unit 5 Test Review • 51. True or False • The formula SO 4 -2 stands for sulfur oxide. False

Unit 5 Test Review • 52. True or False • An atoms is chemically unstable when its outer energy level contains all the electrons it can hold. False

Unit 5 Test Review • 53. True or False • An element’s oxidation number indicates how many electrons the element must gain, lose, or share to become stable. True

Unit 5 Test Review • 54. True or False • In the binary ionic compound lithium iodide, Li. I, iodine would form the anions. True

Unit 5 Test Review • 55. True or False • The chemical formula for calcium chloride, Ca. Cl 2, shows that the compound contains two calcium ions for every chloride ion. False

Unit 5 Test Review • 56. True or False • KBr is the formula for an ionic compound. The fact that neither symbol is followed by a subscript means that there is a one-to-one ratio of ions in the compound. True

Unit 5 Test Review • 57. True or False • In sodium chloride, Na. Cl, the sodium ions are the cations. True

Unit 5 Test Review • 58. True or False • In the binary ionic compound potassium bromide, KBr, the element that forms cations is bromine. False

Unit 5 Test Review (BONUS) • 59. Match the following. You must get ALL of them right to get the points. 1. have a charge of -1 2. do not react 3. have 6 valence electrons 4. have 2 valence electrons 5. have a charge of +1 a. alkali metals b. alkaline earth metals c. group 16 1. d d. halogens 2. e 3. c e. noble gases 4. b 5. a