Physical Science Applications in Agriculture Unit Physical Science



















- Slides: 30

Physical Science Applications in Agriculture Unit Physical Science Systems

Problem Area Agricultural Production Systems

Testing Common Substances for p. H Lesson

Ever see limestone being applied on a grower’s field? • What is so important about this powdery substance? • What does it do for the soil? • Does the composition or type of limestone (liquid, dry, palletized) make a difference in its effectiveness? . acadianashell. com/OFFLOADING 3. JPG

Which plant looks healthier? • Examine the pictures or actual plants that have received different levels of fertilizer. • Which plant looks healthier? • Which plant would produce better and more product? • How much fertilizer do they apply to get optimum growth of our vegetable plants? • Is there a way to determine this? How?

Learning Objectives 1. Define p. H and discuss its role in plant nutrition. 2. Explain how soils become acidic. 3. Explain how soil p. H is measured. 4. Explain why lime is applied to acid soils. 5. Discuss the effectiveness of lime on acidic soils.

Terms • • Acid Calcium carbonate equivalent Cation exchange capacity Lime requirement Percent base saturation p. H scale Soil p. H

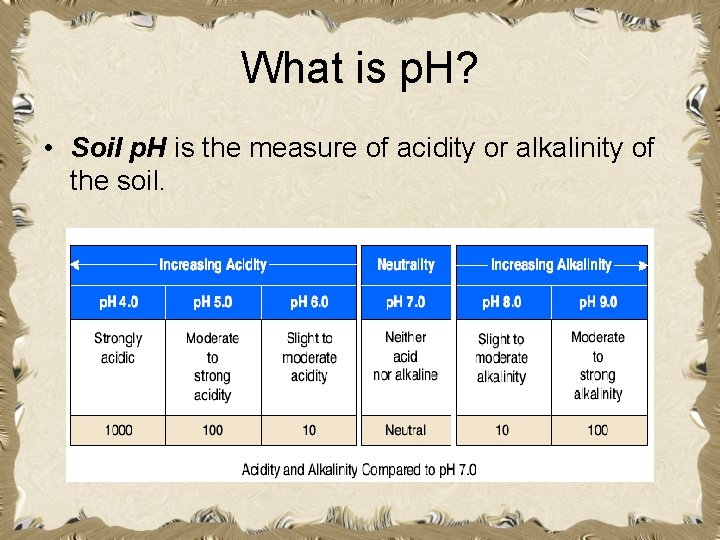
What is p. H? • Soil p. H is the measure of acidity or alkalinity of the soil.

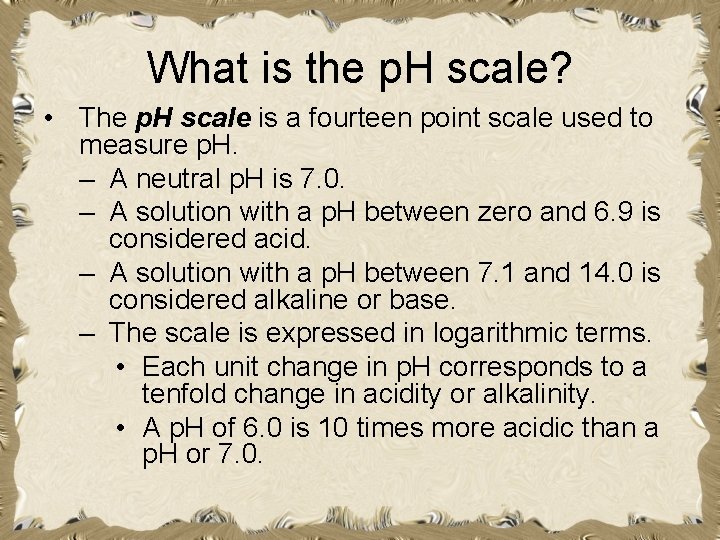
What is the p. H scale? • The p. H scale is a fourteen point scale used to measure p. H. – A neutral p. H is 7. 0. – A solution with a p. H between zero and 6. 9 is considered acid. – A solution with a p. H between 7. 1 and 14. 0 is considered alkaline or base. – The scale is expressed in logarithmic terms. • Each unit change in p. H corresponds to a tenfold change in acidity or alkalinity. • A p. H of 6. 0 is 10 times more acidic than a p. H or 7. 0.

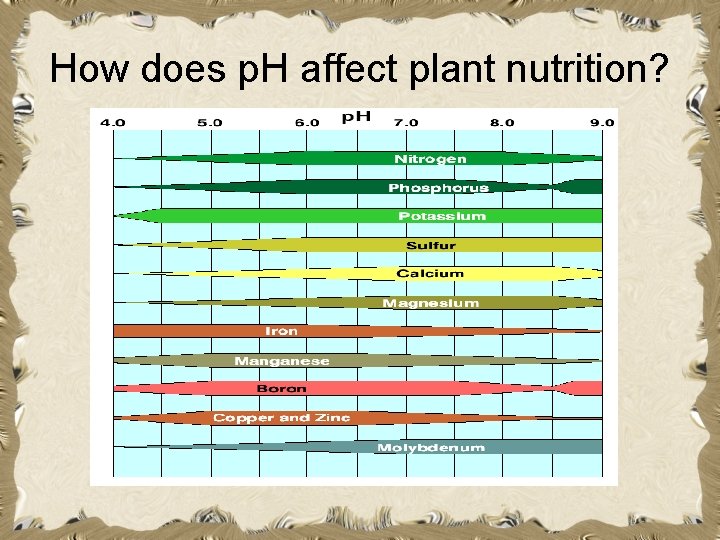
How does p. H affect plant nutrition? • The p. H value of soil is important to agriculturalists because certain nutrients become unavailable to plants if the p. H value is too high or too low. • The amount of nutrients that are available is dependent upon soil p. H.

How does p. H affect plant nutrition?

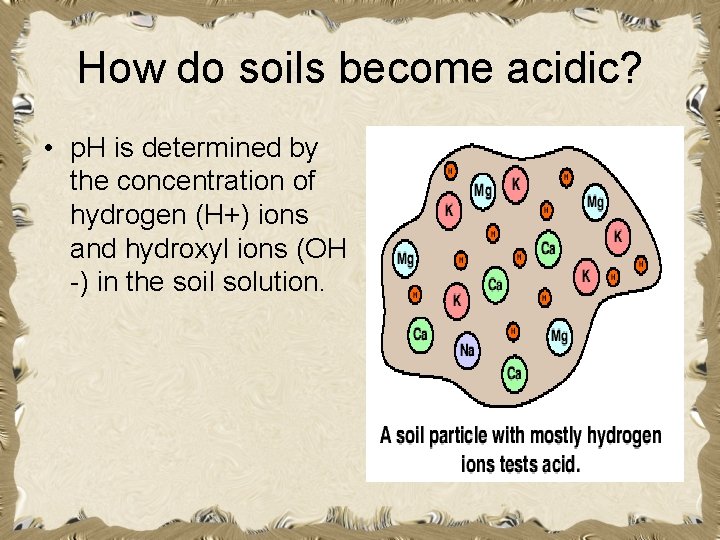
How do soils become acidic? • p. H is determined by the concentration of hydrogen (H+) ions and hydroxyl ions (OH -) in the soil solution.

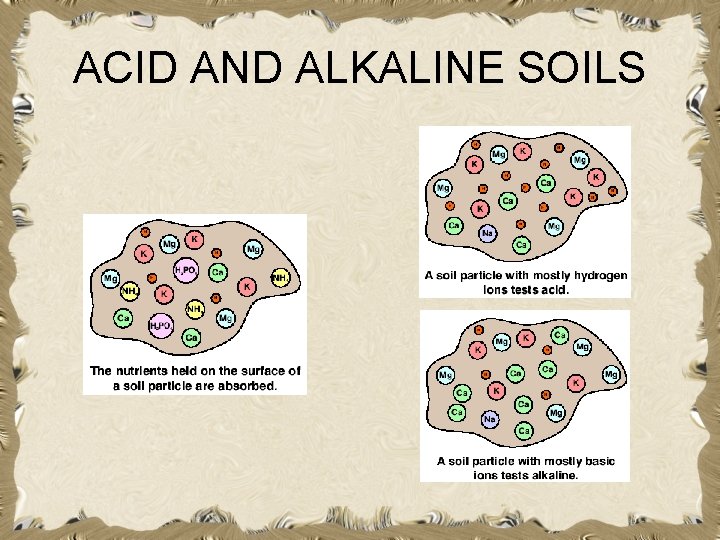
How do soils become acidic? • A sample of pure water has an equal number of H+ and OH- and is neutral. • An acid is a substance that releases hydrogen ions. • When saturated with H+, a soil behaves as a weak acid. • The more H+ held on the exchange complex, the greater the soil’s acidity.

Several factors influence soil p. H. • Soil organic matter is continuously being decomposed by micro organisms into organic acids, carbon dioxide, and water, forming carbonic acid. • Carbonic acid reacts with Ca and Mg carbonates in the soil to form more soluble bicarbonates, which are leached away, leaving the soil more acid.

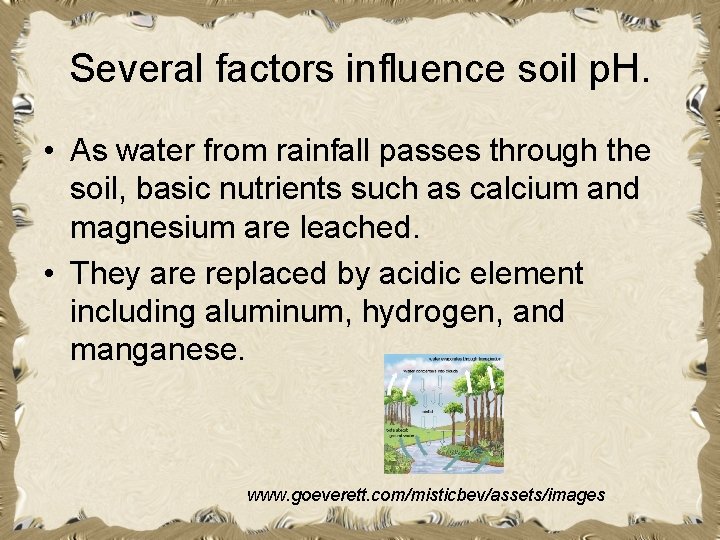
Several factors influence soil p. H. • As water from rainfall passes through the soil, basic nutrients such as calcium and magnesium are leached. • They are replaced by acidic element including aluminum, hydrogen, and manganese. www. goeverett. com/misticbev/assets/images

Several factors influence soil p. H. • Soils formed under forest vegetation tend to be more acidic than those developed under grasslands. www. itc. nl/personal/. . . / t 1765 e 0 s. htm

Several factors influence soil p. H. • Soils often become more acidic when crops are harvested because bases are • removed. • Legumes generally contain higher levels of bases than grasses. • Legumes also release H+ ions into their rhizosphere when actively fixing atmospheric N.

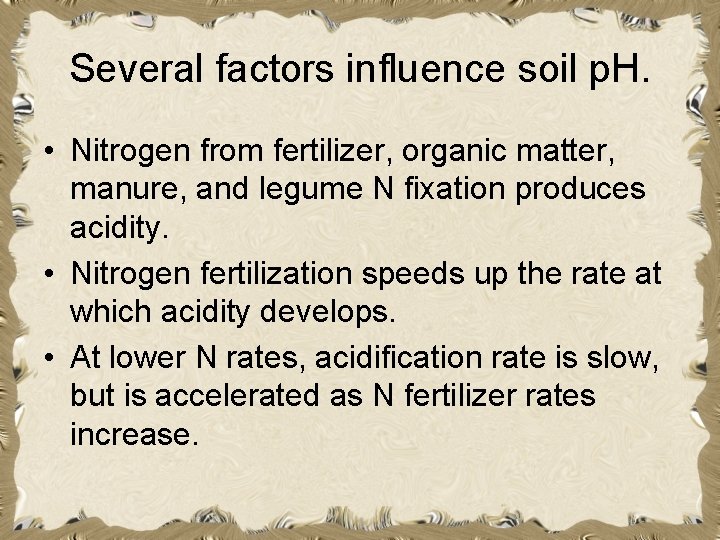
Several factors influence soil p. H. • Nitrogen from fertilizer, organic matter, manure, and legume N fixation produces acidity. • Nitrogen fertilization speeds up the rate at which acidity develops. • At lower N rates, acidification rate is slow, but is accelerated as N fertilizer rates increase.

ACID AND ALKALINE SOILS

How is soil p. H measured? • The two most commonly accepted methods of measuring soil p. H are indicator dyes and the p. H meter. ltpwww. gsfc. nasa. gov. . . globe/pvg/19 -5 ph. jpg

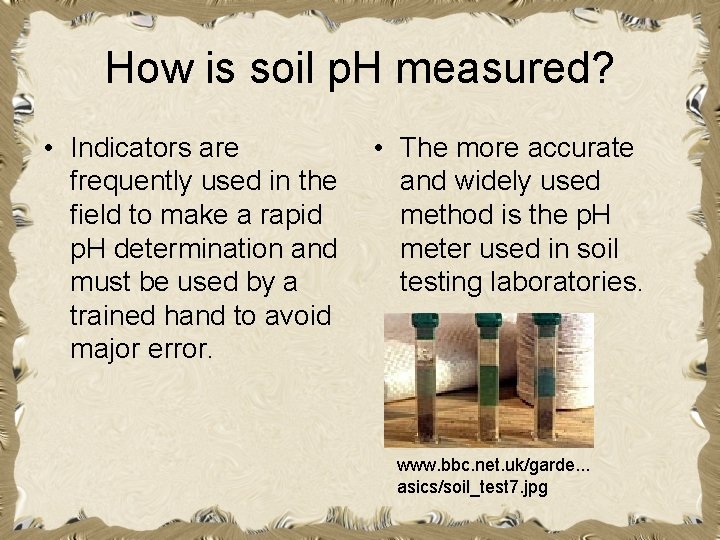
How is soil p. H measured? • Indicators are frequently used in the field to make a rapid p. H determination and must be used by a trained hand to avoid major error. • The more accurate and widely used method is the p. H meter used in soil testing laboratories. www. bbc. net. uk/garde. . . asics/soil_test 7. jpg

Why is lime applied to acidic soils? • Since various plants require different p. H levels for optimum growth, growers must attempt to adjust soil p. H to suit the crop or plant being grown. • This involves the use of limestone to raise p. H or the use of alum to lower p. H. – Lime requirement is the amount of agricultural limestone needed to establish the desired p. H range.

Why is lime applied to acidic soils? • Soil p. H is an excellent single indicator of soil acidity, it does not determine lime requirement. • Lime requirement of a soil is not only related to the p. H but also to its buffer capacity or cation exchange capacity. – Cation exchange capacity is the total number of exchangeable cations, an ion with a positive charge, a soil can adsorb. – The relative amount of the cation exchange capacity filled with basic cations is called percent base saturation. – Soil p. H is a measure of the percent base saturation.

Why is lime applied to acidic soils? • Lime replaces hydrogen and aluminum on the cation exchange sites with calcium and changes hydrogen ions to water. www. imdexminerals. com. au/ag. asp

Factors to figure how much lime is required 1. 2. 3. 4. the present p. H the desired p. H the cation exchange capacity of the soil the liming material to be used www. hort. wisc. edu/. . . / soiltest/soiltest. htm

The effectiveness of lime on soil. • The effectiveness of lime depends on: – Purity – Fineness – Rate it dissolves • Measured as the calcium carbonate equivalent

What determines the effectiveness of lime on acid soils? • The neutralizing power of lime depends upon its purity, measured as the calcium carbonate equivalent. – Neutralizing values of all liming materials are determined by comparing them to the neutralizing value of pure claim carbonate. – Setting the neutralizing value of calcium carbonate at 100, a value for other materials can be assigned.

What determines the effectiveness of lime on acid soils? • When a given quantity of lime is mixed with the soil, its reaction rate and degree of reactivity are affected by particle size. 1. Coarse live particles react more slowly and less fully. 2. Fine lime particles react more rapidly and much more completely. 3. Cost of lime increases with the fineness of grind. 4. The goal is a material that requires a minimum of grinding, yet contains enough fine material to cause a rapid p. H change.

Other important factors determining the effectiveness of lime 1. Placement for maximum contact with the soil in the tilled layer is essential. 2. Even when properly mixed with the soil, lime will have little effect on p. H if the soil is dry. • Moisture is essential for lime-soil reaction to occur. 3. The full benefit of lime is not seen until the second or third year after application. • Lime does not react with acidic soil very far from the lime particle.

Review/Summary • What is p. H and how does it affect plant nutrition? • How do soils become acidic? • How is soil p. H measured? • Why is lime applied to acidic soils? • What determines the effectiveness of lime on acid soils?
 Physical factors influencing agriculture
Physical factors influencing agriculture Physical factors influencing agriculture
Physical factors influencing agriculture History of farming
History of farming Unit 6 review questions
Unit 6 review questions Six branches of science
Six branches of science Natural and physical science
Natural and physical science That's my favorite
That's my favorite Physical devices of a computer
Physical devices of a computer Elements of a computer
Elements of a computer Synchrotron radiation
Synchrotron radiation Novel data science applications
Novel data science applications Vital villages thriving towns introduction
Vital villages thriving towns introduction Circular agriculture
Circular agriculture Hunters and gatherers
Hunters and gatherers Heisa and leisa
Heisa and leisa Slash and burn agriculture synonym
Slash and burn agriculture synonym Safety colors in agriculture
Safety colors in agriculture Agriculture hearths
Agriculture hearths Crown plants examples
Crown plants examples Edaphic factors affecting crop production
Edaphic factors affecting crop production Paid internships in germany
Paid internships in germany What are the ict tools used in agriculture
What are the ict tools used in agriculture Nuclear institute for agriculture and biology
Nuclear institute for agriculture and biology 10 importance of agriculture
10 importance of agriculture Is the component of agriculture renewable action plan
Is the component of agriculture renewable action plan Muhammad nawaz shareef university of agriculture
Muhammad nawaz shareef university of agriculture Ministry of agriculture, water and forestry directorates
Ministry of agriculture, water and forestry directorates Atmis.kilimo
Atmis.kilimo Ministry of agriculture veterinary services division
Ministry of agriculture veterinary services division Leisa agriculture
Leisa agriculture Logical framework approach
Logical framework approach