Physical Science Unit 6 Test Review Game Unit


















































- Slides: 56

Physical Science Unit 6 Test Review Game

Unit 6 Test Review Game 1. Which of the following is a balanced chemical equation for the synthesis of Na. Br from Na and Br 2? • A. 2 Na + Br 2 Na. Br • B. 2 Na + Br 2 2 Na. Br • C. Na + Br 2 Na. Br • D. Na + Br 2 2 Na. Br B

Unit 6 Test Review Game 2. What type of reaction is KCl. O 3 KCl + O 2 • A. combustion • B. double displacement • C. decomposition • D. synthesis • E. single replacement C

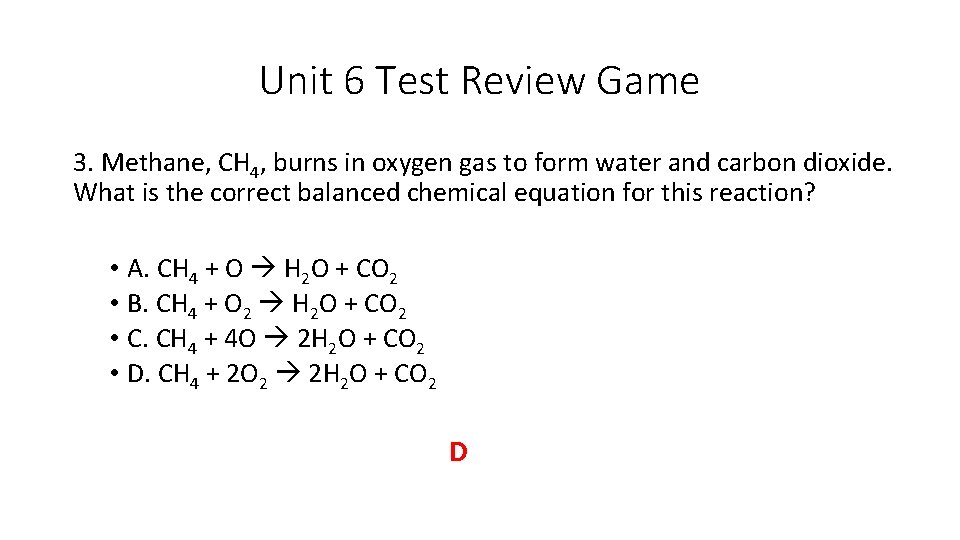
Unit 6 Test Review Game 3. Methane, CH 4, burns in oxygen gas to form water and carbon dioxide. What is the correct balanced chemical equation for this reaction? • A. CH 4 + O H 2 O + CO 2 • B. CH 4 + O 2 H 2 O + CO 2 • C. CH 4 + 4 O 2 H 2 O + CO 2 • D. CH 4 + 2 O 2 2 H 2 O + CO 2 D

Unit 6 Test Review Game 4. Numbers that precede symbols and formulas in a chemical equation are ______. • A. subscripts • B. superscripts • C. coefficients • D. catalysts C

Unit 6 Test Review Game 5. What type of reaction is 2 Mg + O 2 2 Mg. O • A. synthesis • B. decomposition • C. double displacement • D. single replacement • E. combustion A or E

Unit 6 Test Review Game 6. What type of reaction is Pb(NO 3)2 + 2 KI Pb. I 2 + 2 KNO 3? • A. single replacement • B. double displacement • C. combustion • D. decomposition • E. synthesis B

Unit 6 Test Review Game 7. Each substance to the right of the arrow in a chemical equation is a(n) ________. • A. reactant • B. catalyst • C. inhibitor • D. product D

Unit 6 Test Review Game 8. Which of the following is the correct balanced equation of: Cu + HNO 3 Cu(NO 3)2 + NO 2 + H 2 O • A. Cu + 4 HNO 3 Cu(NO 3)2 + 2 NO 2 + 2 H 2 O • B. Cu + 4 HNO 3 Cu(NO 3)2 + 4 NO 2 + 2 H 2 O • C. Cu + HNO 3 Cu(NO 3)2 + NO 2 + H 2 O • D. Cu + 2 HNO 3 Cu(NO 3)2 + 2 NO 2 + H 2 O A

Unit 6 Test Review Game 9. Which of the following does NOT state what the arrow means in a chemical reaction? • A. conserves • B. produces • C. yields • D. forms A

Unit 6 Test Review Game 10. The substances that undergo change in a chemical reaction are called • A. products. • B. reactants. • C. elements. • D. coefficients. B

Unit 6 Test Review Game 11. Substances that speed up chemical reactions are called _____. • A. inhibitors • B. products • C. endothermics • D. catalysts D

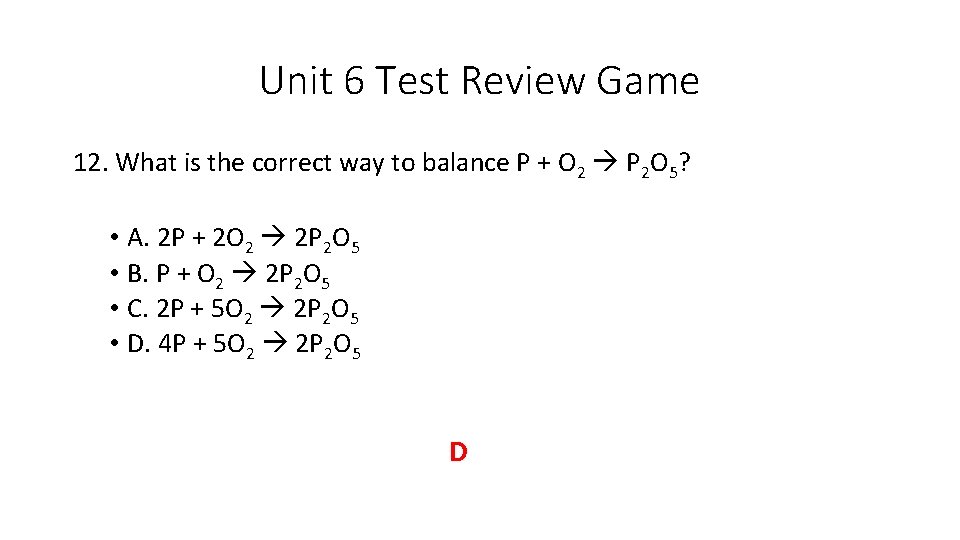
Unit 6 Test Review Game 12. What is the correct way to balance P + O 2 P 2 O 5? • A. 2 P + 2 O 2 2 P 2 O 5 • B. P + O 2 2 P 2 O 5 • C. 2 P + 5 O 2 2 P 2 O 5 • D. 4 P + 5 O 2 2 P 2 O 5 D

Unit 6 Test Review Game 13. What type of reaction is Ca. Br 2 + Na. CO 3 Ca(CO 3)2 + Na. Br? • A. combustion • B. decomposition • C. synthesis • D. single replacement • E. double displacement E

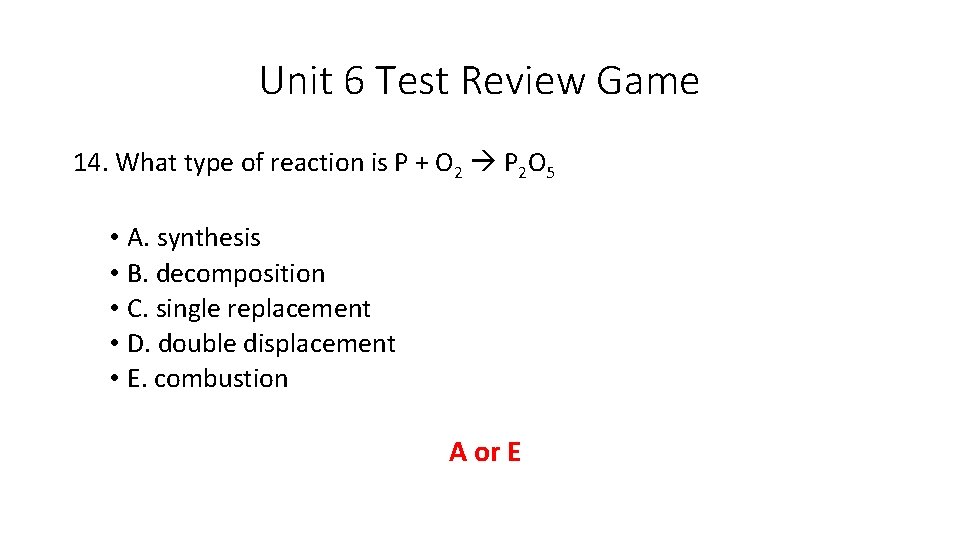
Unit 6 Test Review Game 14. What type of reaction is P + O 2 P 2 O 5 • A. synthesis • B. decomposition • C. single replacement • D. double displacement • E. combustion A or E

Unit 6 Test Review Game 15. What is the correct way to balance KCl. O 3 KCl + O 2? • A. 2 KCl. O 3 KCl + O 2 • B. 2 KCl. O 3 2 KCl + 3 O 2 • C. 2 KCl. O 3 2 KCl + O 2 • D. KCl. O 3 KCl + O 2 B

Unit 6 Test Review Game 16. What is the correct way to balance Ca. Br 2 + Na. CO 3 Ca(CO 3)2 + Na. Br? • A. Ca. Br 2 + Na. CO 3 Ca(CO 3)2 + 2 Na. Br • B. 2 Ca. Br 2 + 2 Na. CO 3 Ca(CO 3)2 + Na. Br • C. 2 Ca. Br 2 + Na. CO 3 2 Ca(CO 3)2 + Na. Br • D. Ca. Br 2 + 2 Na. CO 3 Ca(CO 3)2 + 2 Na. Br D

Unit 6 Test Review Game 17. If there are 4 hydrogen atoms on the left side of an equation, then how many hydrogen atoms must there be on the right side in order to balance this reaction? • A. at least 2 • B. 8 • C. it does not matter • D. 4 D

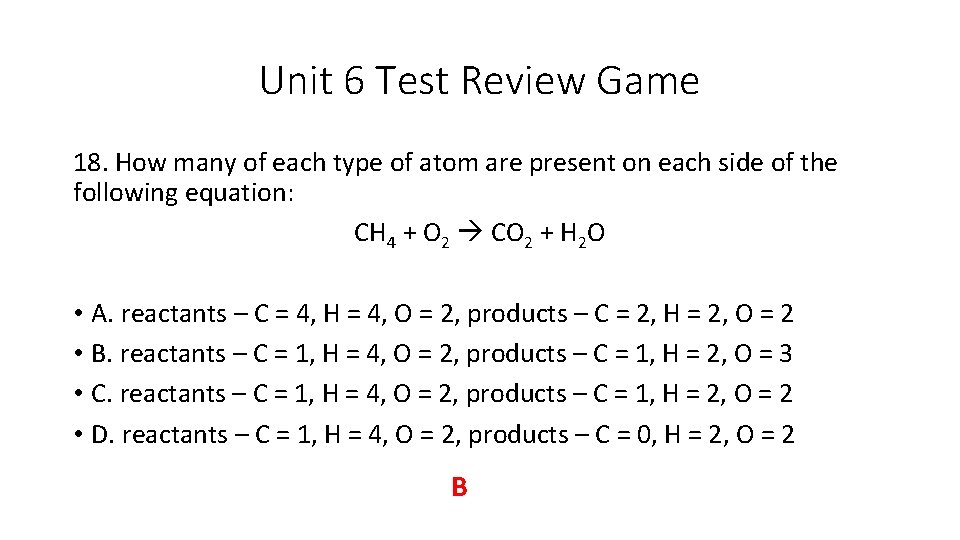
Unit 6 Test Review Game 18. How many of each type of atom are present on each side of the following equation: CH 4 + O 2 CO 2 + H 2 O • A. reactants – C = 4, H = 4, O = 2, products – C = 2, H = 2, O = 2 • B. reactants – C = 1, H = 4, O = 2, products – C = 1, H = 2, O = 3 • C. reactants – C = 1, H = 4, O = 2, products – C = 1, H = 2, O = 2 • D. reactants – C = 1, H = 4, O = 2, products – C = 0, H = 2, O = 2 B

Unit 6 Test Review Game 19. How do you balance the number of hydrogen atoms in the following equation: CH 4 + O 2 CO 2 + H 2 O • A. Place a coefficient of 2 in front of water on the right side. • B. Place a coefficient of 2 in front of the methane on the left side and a coefficient of 2 in front of water on the right side. • C. Place a coefficient of 4 in front of water on the right side. • D. it is already balanced A

Unit 6 Test Review Game 20. True or False • The general formula for a synthesis reaction is AB A + B. False

Unit 6 Test Review Game 21. True or False • A subscript is the number that appears before a formula in a chemical reaction. False

Unit 6 Test Review Game 22. True or False • C 3 H 8 + O 2 CO 2 + H 2 O is a combustion reaction. True

Unit 6 Test Review Game 23. True or False • 3 H 2 + N 2 2 NH 3 is a balanced equation. True

Unit 6 Test Review Game 24. True or False • C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O is NOT balanced. False

Unit 6 Test Review Game 25. What type of reaction is the following? AB A + B A. B. C. D. Synthesis Decomposition Single replacement Double displacement B

Unit 6 Test Review Game 26. What type of reaction is the following? A + BC AC + B • A. Synthesis • B. Decomposition • C. Single Replacement • D. Double Displacement C

Unit 6 Test Review Game 27. A salt is dissolved in water, which has a boiling point of 100 o. C. The boiling point of the solution will be • A. greater than 100 o. C • B. less than 100 o. C • C. equal to 100 o. C • D. equal to 0 o. C A

Unit 6 Test Review Game 28. In order for a solution to form, • A. one substance must dissolve in another. • B. a solid must dissolve in a liquid. • C. the solvent must be water. • D. a gas must dissolve in a liquid. A

Unit 6 Test Review Game 29. A solution that contains more solute than it would normally hold at that temperature is said to be • A. saturated • B. unsaturated • C. supersaturated • D. concentrated C

Unit 6 Test Review Game 30. During the formation of a solution, energy is • A. either released or absorbed. • B. neither released or absorbed. • C. released only. • D. absorbed only. A

Unit 6 Test Review Game 31. One way to determine the degree of saturation of a solid-liquid solution is to drop a crystal of the solute into the solution. If the crystal sits at the bottom of the container, the solution is • A. saturated. • B. unsaturated. • C. supersaturated. • D. concentrated. A

Unit 6 Test Review Game 32. The solubility of carbon dioxide gas in water can be increased by • A. increasing the temperature. • B. agitating the mixture. • C. increasing the pressure. • D. using more solvent. C

Unit 6 Test Review Game 33. All materials that are considered to be insoluble will dissolve in a solvent to a very small extent. The rate of dissolving of a rock in a streambed would not be increased by which of the following? • A. breaking the rock into smaller pieces • B. moving the rock to a faster-moving current • C. moving the rock to warmer water • D. reducing the exposure of the rock to the water D

Unit 6 Test Review Game 34. The maximum amount of a solute that will dissolve in a certain amount of solvent at a given temperature is that solute’s • A. solubility. • B. concentration. • C. degree of saturation. • D. rate of solution. A

Unit 6 Test Review Game 35. A student dissolved equal amounts of salt in equal amounts of warm water, room-temperature water, and ice water. Which of the following is true? • A. The salt dissolved most quickly in the warm water. • B. The salt dissolved most quickly in the room-temperature water. • C. The salt dissolved most quickly in the ice water. • D. None of the above A

Unit 6 Test Review Game 36. A base is defined as a compound that produces • A. hydroxide ions in solution. • B. hydrogen ions in solution. • C. hydronium ions in solution. • D. sodium ions in solution. A

Unit 6 Test Review Game 37. Which of the following is NOT a property of an acid? • A. tastes sour • B. usually reacts with a metal • C. changes the color of an indicator • D. feels slippery D

Unit 6 Test Review Game 38. Which of the following is NOT a common property of bases? • A. feels slippery • B. tastes bitter • C. reacts with metals • D. changes color of indicators C

Unit 6 Test Review Game 39. A compound has a p. H of 6 in solution, where very little of it has ionized. The compound is a • A. weak base. • B. strong base. • C. weak acid. • D. strong acid. C

Unit 6 Test Review Game 40. When sugar dissolves in water, water is the _______ and sugar is the _______. Solvent, solute

Unit 6 Test Review Game 41. An acid produces _______ when it is dissolved in water. H+ ions or hydronium ions (H 3 O+)

Unit 6 Test Review Game 42. Table salt is _________ soluble in cold water than it is in hot water. less

Unit 6 Test Review Game 43. What type of nuclear decay releases energy but not a particle? • A. alpha decay • B. beta decay • C. gamma decay • D. electron decay C

Unit 6 Test Review Game 44. What is the process in which an unstable atomic nucleus emits charged particles or energy or both? • A. radioactivity • B. oxidation • C. decomposition • D. none of the above A

Unit 6 Test Review Game 45. Which of the following statements is true? • A. Chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. • B. Nuclear decay rates vary with conditions, but chemical reaction rates do not. • C. Both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction. • D. Neither chemical reactions rates nor nuclear decay rates vary with the conditions of the reaction. A

Unit 6 Test Review Game 46. Many people work near a source of nuclear radiation. To detect the amount of exposure they have to radiation, they most likely will use a • A. Geiger counter. • B. film badge. • C. radon kit. • D. lead shield. B

Unit 6 Test Review Game 47. The half-life of tritium, of hydrogen-3, is 12. 32 years. After about 24. 6 years, how much of a sample of tritium will remain unchanged? • A. 1/8 • B. 1/4 • C. 1/3 • D. 1/2 B

Unit 6 Test Review Game 48. The half-life of a radioisotope is the amount of time it takes for • A. half the sample to decay. • B. all the sample to decay. • C. the age of an artifact to be calculated. • D. detectable radiation to be absorbed by a sample. A

Unit 6 Test Review Game 49. Which of the following particles is smaller than the rest? • A. electron • B. proton • C. neutron • D. alpha particle A

Unit 6 Test Review Game 50. During nuclear fission, great amounts of energy are produced from • A. very small amounts of mass. • B. tremendous amounts of mass. • C. a series of chemical reactions. • D. particle accelerators. A

Unit 6 Test Review Game 51. A rock containing a radioisotope is broken down into a powder, greatly increasing its surface area. At the same time, the temperature of the sample is lowered 25 o. C. The half-life of the radioisotope will ______. Stay the same

Unit 6 Test Review Game 52. A sample of a radioisotope had a mass of 100. 0 g. After exactly 24 days, 6. 25 g of the sample remain unchanged. The half-life of the isotope is _____ days. 6

Unit 6 Test Review Game 53. In nuclear reactions, ______ is converted into energy. mass

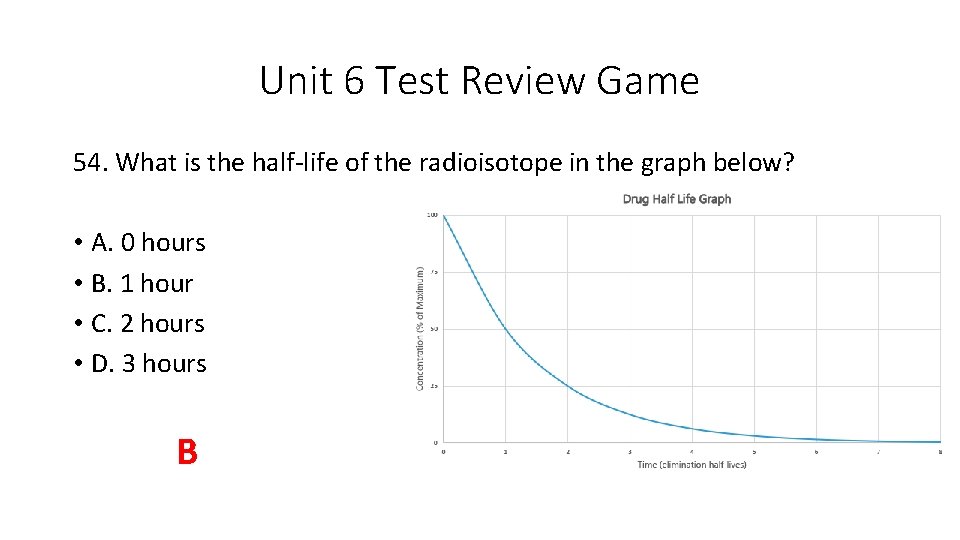
Unit 6 Test Review Game 54. What is the half-life of the radioisotope in the graph below? • A. 0 hours • B. 1 hour • C. 2 hours • D. 3 hours B

Unit 6 Test Review Game 55. What is the BEST explanation for the reaction below? • A. It is fission because smaller particles are the products. • B. It is fusion because smaller particles are the products. • C. It is fission because a larger particle is the product. • D. It is fusion because a larger particle is the product. D