Pathogenesis and treatment of chronic rhinosinusitis 2018 11




















- Slides: 43

Pathogenesis and treatment of chronic rhinosinusitis 葉德輝醫師 臺大醫院耳鼻喉部 2018 -11 -03

European position paper on rhinosinusitis and nasal polyps (EPOS 2012) • Rhinosinusitis in adults is defined as: inflammation of the nose and the paranasal sinuses characterized by two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip): - ± facial pain/pressure - ± reduction or loss of smell and either endoscopic signs of: - nasal polyps, and/or - mucopurulent discharge primarily from middle meatus and/or oedema/mucosal obstruction primarily in middle meatus • It was further classified as Acute (< 12 weeks, complete resolution of symptoms) and Chronic (≥ 12 weeks without complete resolution of symptoms) forms. USA Chronic rhinosinusitis (CRS) is a disease of inflammation of the nose and paranasal sinuses and upper airways characterized by 12 weeks of persistent symptoms including congestion, stuffiness, nasal discharge, pain or facial pressure, impairment or loss of the sense of smell (anosmia), cough, and fatigue.

慢性鼻竇炎如何發生 • An inflammatory condition • Lasts 12 weeks or longer • Heterogeneous group

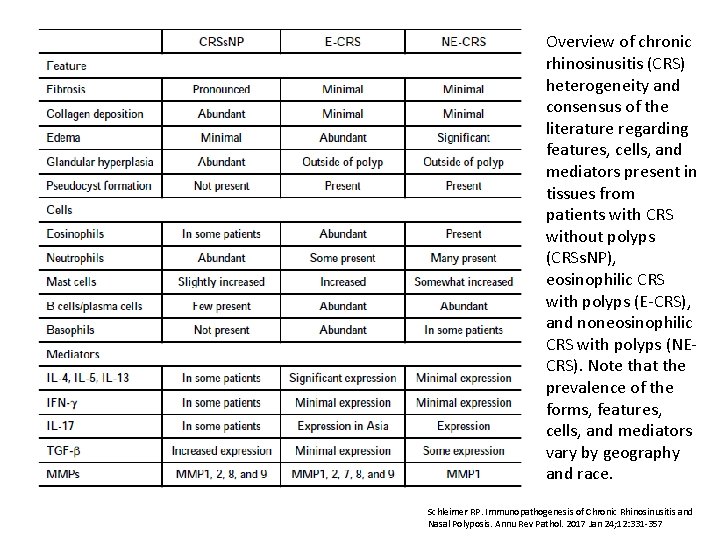
Overview of chronic rhinosinusitis (CRS) heterogeneity and consensus of the literature regarding features, cells, and mediators present in tissues from patients with CRS without polyps (CRSs. NP), eosinophilic CRS with polyps (E-CRS), and noneosinophilic CRS with polyps (NECRS). Note that the prevalence of the forms, features, cells, and mediators vary by geography and race. Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol. 2017 Jan 24; 12: 331 -357

1. Anatomical characteristics 2. Epithelium as epimmunome 3. Immune perspectives 4. Microbiome study

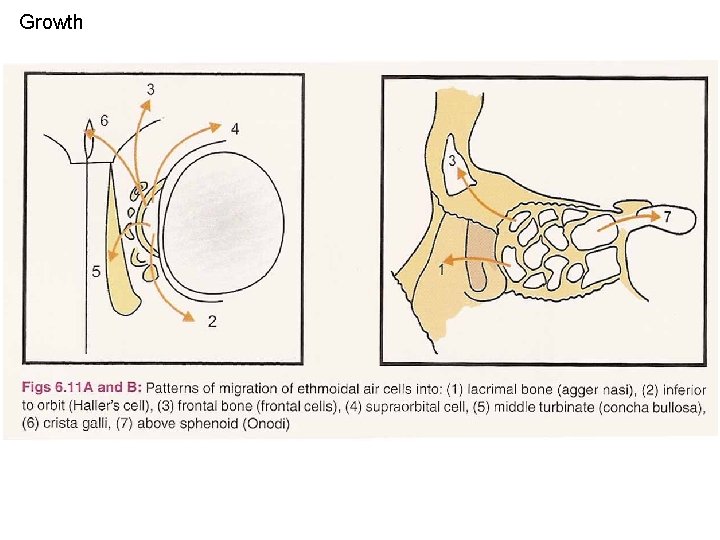
Growth

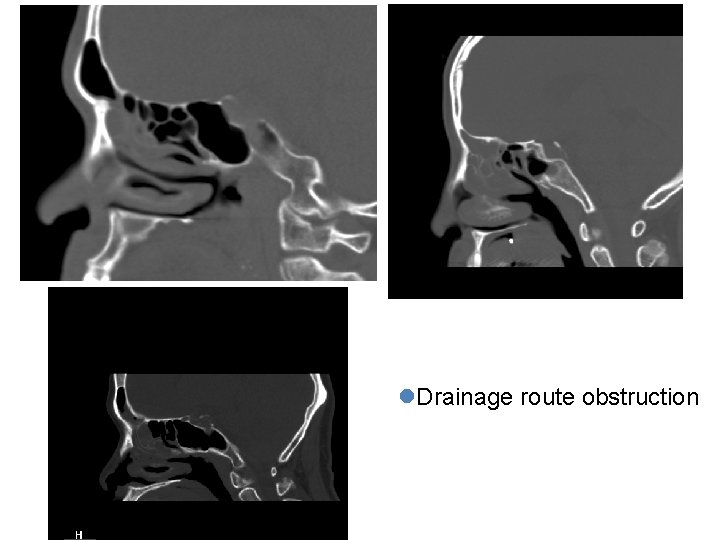
l. Drainage route obstruction

Treatment strategy of rhinosinusitis • Anatomical factors - surgical correction – Occluded ostium (concha bullosa or paradoxical bending of middle turbinate, role of uncinate process, Haller’s cell) – Deep seated recess (high position of maxillary sinus ostium), etc. • Host factors – medical treatment – Immunity factors: allergy, immotile cilia, cystic fibrosis – Environmental factors: pollution, occupational exposure • Pathogen factors - antibiotics – Fungus, resistant strain of bacteria—MRSA, Strep. pneumonia, Pseudomonas etc.

Main objects of FESS (Functional Endoscopic Sinus Surgery) • Correct the anatomical flaws in ostiomeatal complex and diseased sinus • Decrease the inflammatory load (inflamed tissue: polyp and bone) • Facilitate healing process with postoperative care (topical use of steroid or saline or other anti -inflammatory medication, air exposure)

Clinically My learning curve of FESS • 1 st decade: advance knowledge of anatomy and avoid complication • 2 nd decade: standardization of surgical procedure • 3 rd decade: identify refractory cases


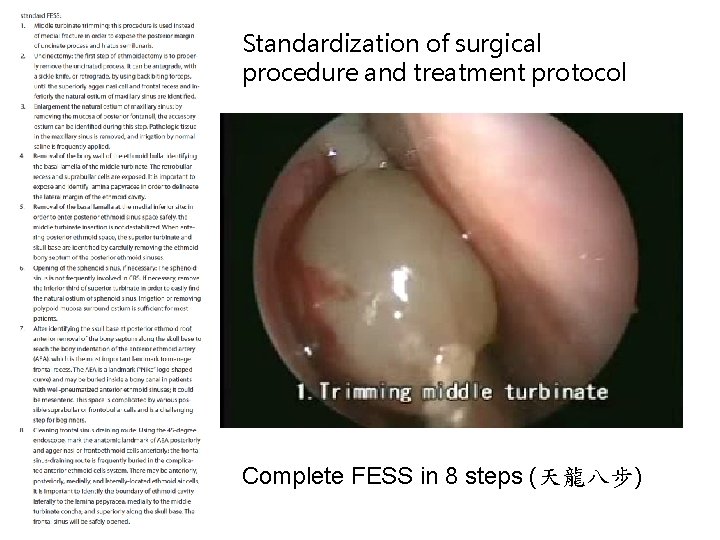
Standardization of surgical procedure and treatment protocol Complete FESS in 8 steps (天龍八步)

Discussion 1. In addition to the classical L-M scoring system, a 2 -dimensional parameter, we suggest that these 2 factors may represent another 3 -dimensional parameter that may indicate the depth of inflammation in CRS in order to evaluate its severity. 2. The sinonasal organ is an expanding air-filled space that grows in a random pattern proven by using a simple computerized equation. The sinus pneumatization process may be considered as using limited material ballooning to occupy the space among the eyeballs, brain, and mouth. This expanding process creates one frontal, maxillary, and sphenoid sinus cell on each side, and more importantly, the complexity of the ethmoid cell system. 3. The bony sinus wall, which confines this sinonasal cavity, represents the boundary and most peripheral lining of this organ. If it is involved in the inflammatory process, it might be considered as one of the dimensions of the depth of disease extent, indicating a more severe form of sinonasal disease.

1. Anatomical characteristics 2. Epithelium as epimmunome 3. Immune perspectives 4. Microbiome study

Nat Immunol. 2010 Aug; 11(8): 656 -65.



1. Disruption of mechanical barrier 2. Epithelial-derived antimicrobial peptides decreas

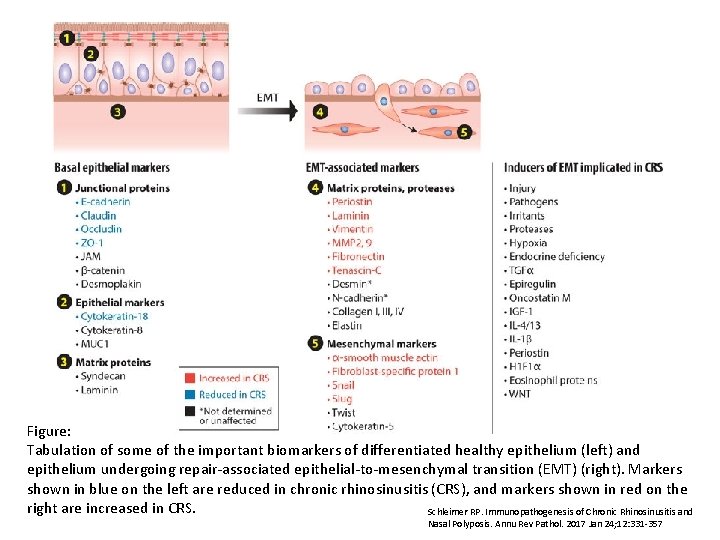
Figure: Tabulation of some of the important biomarkers of differentiated healthy epithelium (left) and epithelium undergoing repair-associated epithelial-to-mesenchymal transition (EMT) (right). Markers shown in blue on the left are reduced in chronic rhinosinusitis (CRS), and markers shown in red on the right are increased in CRS. Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol. 2017 Jan 24; 12: 331 -357

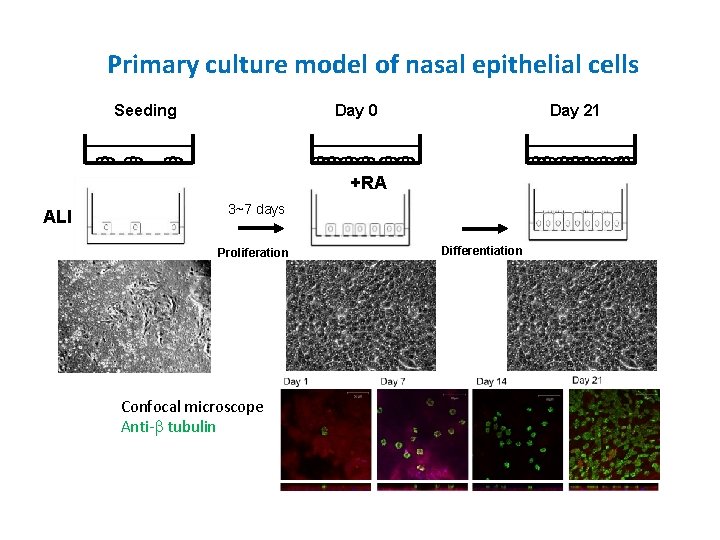
Primary culture model of nasal epithelial cells Seeding Day 0 Day 21 +RA ALI 3~7 days Proliferation Confocal microscope Anti-b tubulin Differentiation

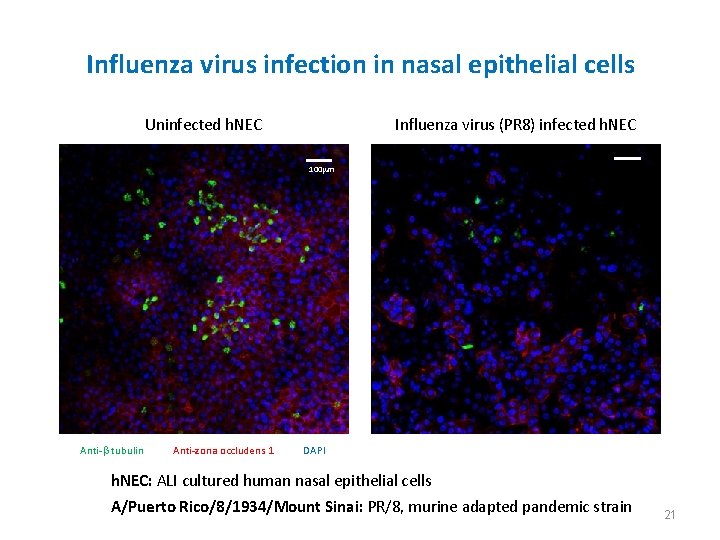
Influenza virus infection in nasal epithelial cells Uninfected h. NEC Influenza virus (PR 8) infected h. NEC 100 mm Anti-b tubulin Anti-zona occludens 1 DAPI h. NEC: ALI cultured human nasal epithelial cells A/Puerto Rico/8/1934/Mount Sinai: PR/8, murine adapted pandemic strain 21

1. Disruption of mechanical barrier 2. Epithelial-derived antimicrobial peptides decrease

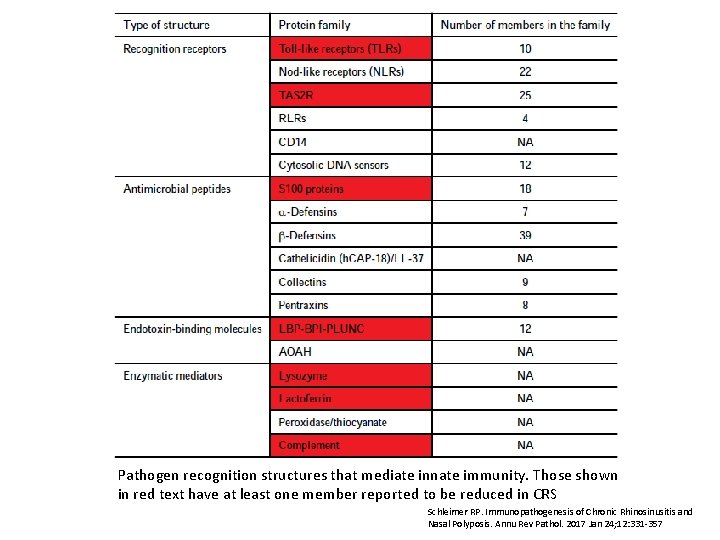
Pathogen recognition structures that mediate innate immunity. Those shown in red text have at least one member reported to be reduced in CRS Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol. 2017 Jan 24; 12: 331 -357

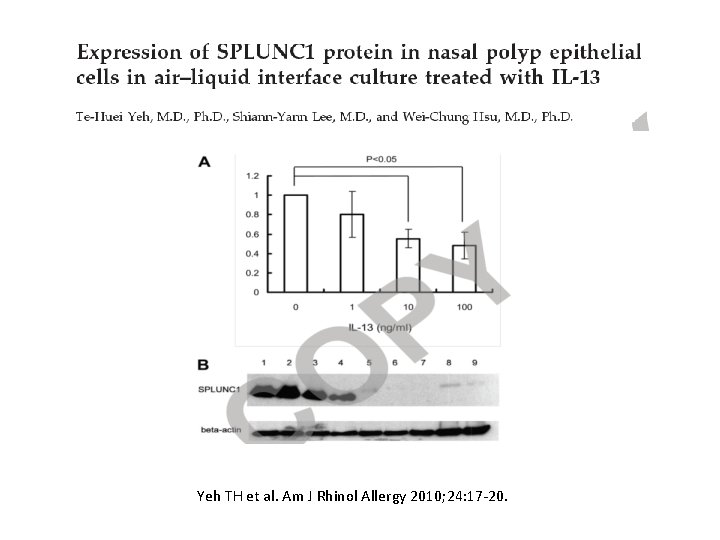
Yeh TH et al. Am J Rhinol Allergy 2010; 24: 17 -20.

1. Anatomical characteristics 2. Epithelium as epimmunome 3. Immune perspectives 4. Microbiome study

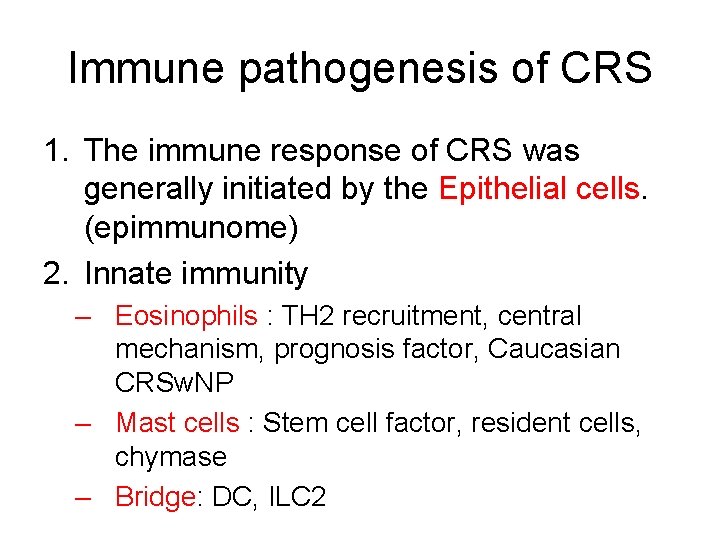
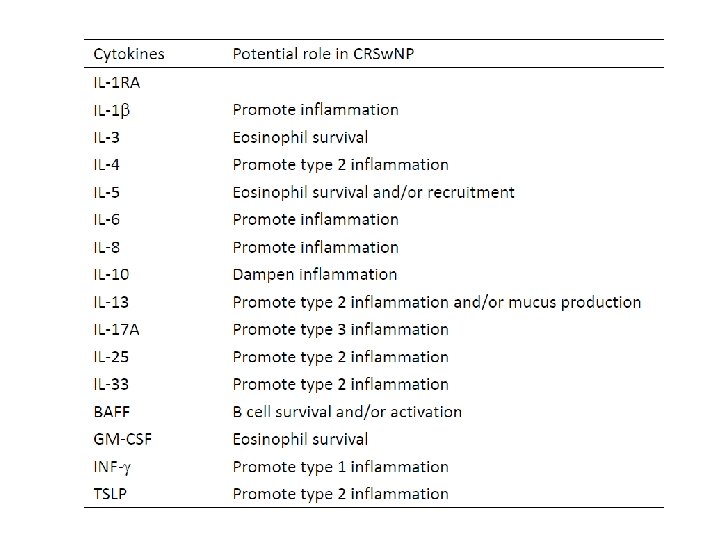
Immune pathogenesis of CRS 1. The immune response of CRS was generally initiated by the Epithelial cells. (epimmunome) 2. Innate immunity – Eosinophils : TH 2 recruitment, central mechanism, prognosis factor, Caucasian CRSw. NP – Mast cells : Stem cell factor, resident cells, chymase – Bridge: DC, ILC 2

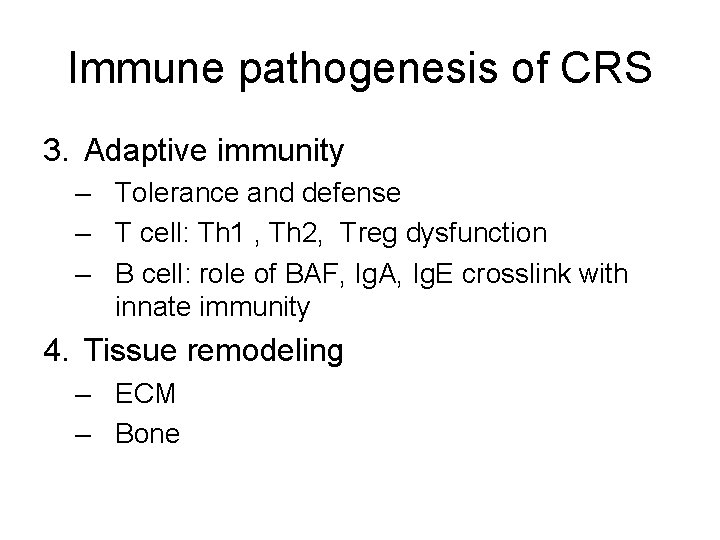
Immune pathogenesis of CRS 3. Adaptive immunity – Tolerance and defense – T cell: Th 1 , Th 2, Treg dysfunction – B cell: role of BAF, Ig. A, Ig. E crosslink with innate immunity 4. Tissue remodeling – ECM – Bone



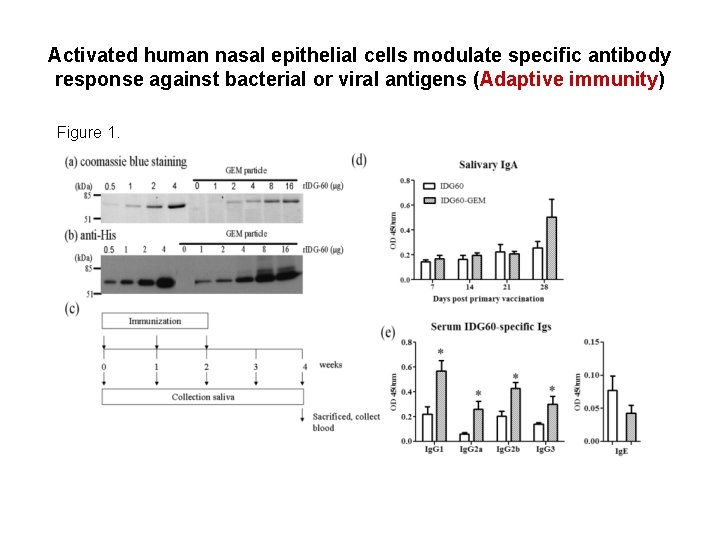
Activated human nasal epithelial cells modulate specific antibody response against bacterial or viral antigens (Adaptive immunity) Figure 1.

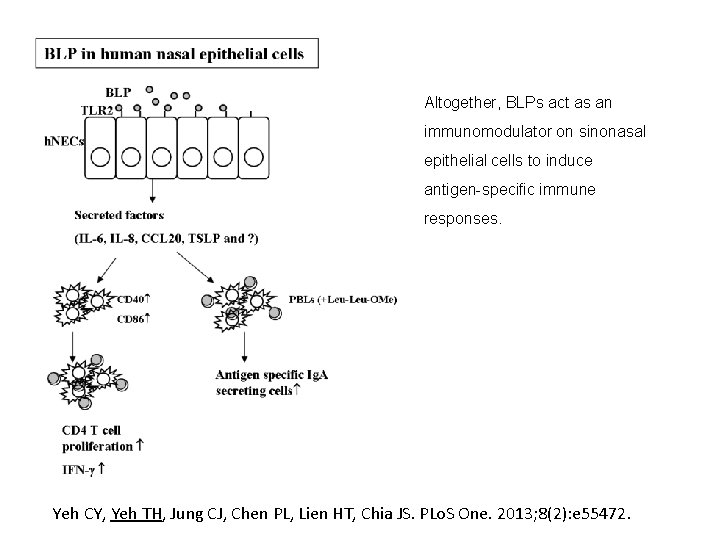
Altogether, BLPs act as an immunomodulator on sinonasal epithelial cells to induce antigen-specific immune responses. Yeh CY, Yeh TH, Jung CJ, Chen PL, Lien HT, Chia JS. PLo. S One. 2013; 8(2): e 55472.

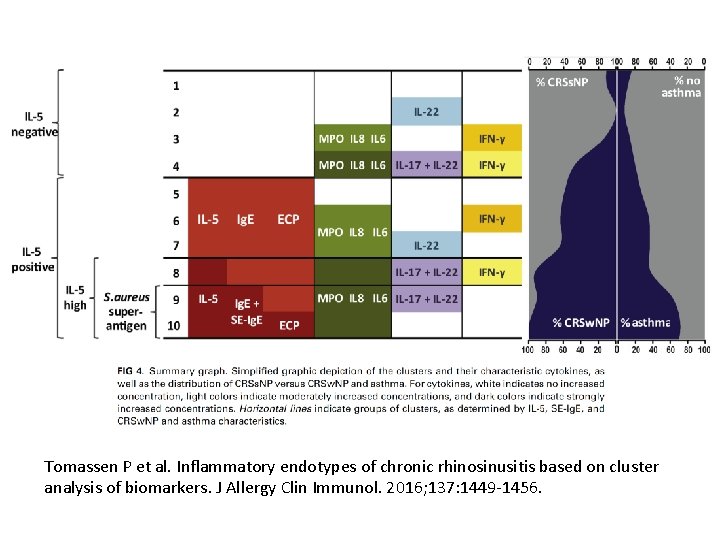
Tomassen P et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016; 137: 1449 -1456.

1. Anatomical characteristics 2. Epithelium as epimmunome 3. Immune perspectives 4. Microbiome study

Microbiome in CRS • Associations are still indecisive: – Initiation and/or progression? – Multiple environmental factors – An epiphenomenon • Why is the microbiome still important? – Strong infectious components – Resident microbes play a role in CRS M. Mahdavinia et al Clin Exp Allergy 2015

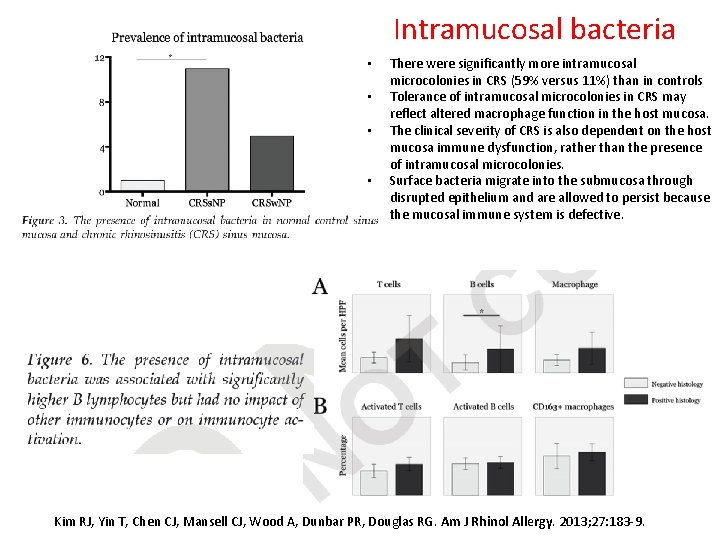
Intramucosal bacteria • • There were significantly more intramucosal microcolonies in CRS (59% versus 11%) than in controls Tolerance of intramucosal microcolonies in CRS may reflect altered macrophage function in the host mucosa. The clinical severity of CRS is also dependent on the host mucosa immune dysfunction, rather than the presence of intramucosal microcolonies. Surface bacteria migrate into the submucosa through disrupted epithelium and are allowed to persist because the mucosal immune system is defective. Kim RJ, Yin T, Chen CJ, Mansell CJ, Wood A, Dunbar PR, Douglas RG. Am J Rhinol Allergy. 2013; 27: 183 -9.

Staphylococcus aureus Superantigen hypothesis • Possible pathogenesis: – Eosinophil – T cell→ Th 2, HLA class II →CRSw. NP – B cell→ local polyclonal Ig. E • Detected in CRSw. NP only • Initiation or progression ? Kent Lam et al. The Etiology and Pathogenesis of Chronic Rhinosinusitis: a Review of Current Hypotheses. Curr Allergy Asthma Rep 2015 July.

Biofilms • Common in CRS patients • S. aureus biofilm • Against – Common bacterial property – Baby shampoo experiment • Important but not major causative Kent Lam et al. Curr Allergy Asthma Rep 2015

Fungal microbiome • The hypothesis (From Mayo Clinic): common airborne fungal elements • Abandoned due to: – Eosinophil!? – In vivo failed – Antifungal treatment failed • Likely play a significant role in a subpopulation of CRS patients Kent Lam et al. Curr Allergy Asthma Rep 2015

Viral microbiome • Rhinovirus in CRS acute exacerbation • The result is scarce and not conclusive • Further investigation needed Kent Lam et al. Curr Allergy Asthma Rep 2015

Summary • Generally not conclusive • Consistent results 1. The microbiome differs 2. Abundance increased • Why the microbiome is still important? 1. Microbiome alter immune response 2. Microbial products 3. Immune system and cell type Kent Lam et al. Curr Allergy Asthma Rep 2015 Abreu NA, Nagalingam NA, Song Y et al. Sci Transl Med 2012

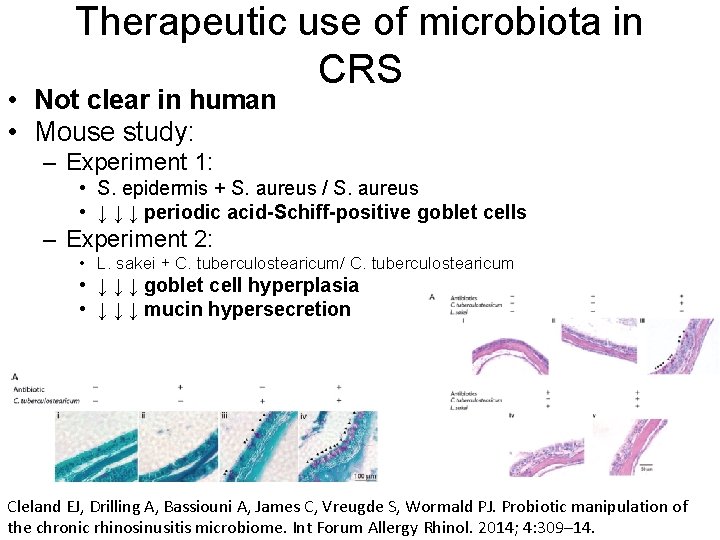
Therapeutic use of microbiota in CRS • Not clear in human • Mouse study: – Experiment 1: • S. epidermis + S. aureus / S. aureus • ↓ ↓ ↓ periodic acid-Schiff-positive goblet cells – Experiment 2: • L. sakei + C. tuberculostearicum/ C. tuberculostearicum • ↓ ↓ ↓ goblet cell hyperplasia • ↓ ↓ ↓ mucin hypersecretion Cleland EJ, Drilling A, Bassiouni A, James C, Vreugde S, Wormald PJ. Probiotic manipulation of the chronic rhinosinusitis microbiome. Int Forum Allergy Rhinol. 2014; 4: 309– 14.

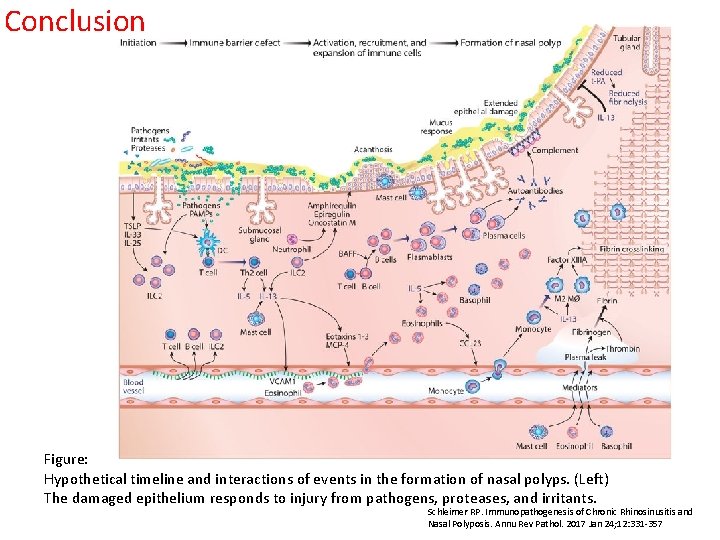
Conclusion Figure: Hypothetical timeline and interactions of events in the formation of nasal polyps. (Left) The damaged epithelium responds to injury from pathogens, proteases, and irritants. Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol. 2017 Jan 24; 12: 331 -357

 Nursing management of pyelonephritis
Nursing management of pyelonephritis Rhinosinusitis
Rhinosinusitis Treatment of cml
Treatment of cml Oncocytoma salivary gland
Oncocytoma salivary gland Renal disease
Renal disease Chronic calculous cholecystitis
Chronic calculous cholecystitis Cholecystitis pathogenesis
Cholecystitis pathogenesis Bacterial pathogenesis
Bacterial pathogenesis Cholecystitis pathophysiology
Cholecystitis pathophysiology Bacterial pathogenesis
Bacterial pathogenesis Tetanus
Tetanus Jaundice pathogenesis
Jaundice pathogenesis Pathogenesis dengue fever
Pathogenesis dengue fever Rabies pathogenesis
Rabies pathogenesis Prepenetration
Prepenetration Baylor canvs
Baylor canvs Left parasternal heave
Left parasternal heave Symptoms liver cirrhosis
Symptoms liver cirrhosis Histoplasma capsulatum pathogenesis
Histoplasma capsulatum pathogenesis Pathophysiology of anemia
Pathophysiology of anemia Mechanism of ischemic stroke
Mechanism of ischemic stroke Pathogenesis of tuberculosis
Pathogenesis of tuberculosis Dada la siguiente secuencia rusia 2018 rusia 2018
Dada la siguiente secuencia rusia 2018 rusia 2018 Cardinal signs of inflammation
Cardinal signs of inflammation Chapter 18 common chronic and acute conditions
Chapter 18 common chronic and acute conditions Morphological patterns of inflammation
Morphological patterns of inflammation How common is leukemia in adults
How common is leukemia in adults Cyst granuloma abscess
Cyst granuloma abscess Angioectasia icd 10
Angioectasia icd 10 Definition of chronic toxicity
Definition of chronic toxicity Flinders model of care
Flinders model of care Who developed the chronic care model
Who developed the chronic care model Earthy look in chronic renal failure
Earthy look in chronic renal failure Chronic granulomatous disease
Chronic granulomatous disease Chronic care model definition
Chronic care model definition Pulp and periapical diseases
Pulp and periapical diseases Types of anemia
Types of anemia Fibromyalgia vs chronic fatigue
Fibromyalgia vs chronic fatigue Stigmata of chronic liver disease
Stigmata of chronic liver disease Reversible and irreversible pulpitis
Reversible and irreversible pulpitis Iceberg phenomenon definition
Iceberg phenomenon definition Hemolysis symptoms
Hemolysis symptoms Chronic unease safety
Chronic unease safety Chronic calculous cholecystitis
Chronic calculous cholecystitis