Overall Survival of Patients with RelapsedRefractory Multiple Myeloma








- Slides: 23

Overall Survival of Patients with Relapsed/Refractory Multiple Myeloma Treated with Carfilzomib, Lenalidomide, and Dexamethasone versus Lenalidomide and Dexamethasone: Final Analysis from the Randomized Phase 3 ASPIRE Trial A. Keith Stewart, 1 David Siegel, 2 Heinz Ludwig, 3 Thierry Facon, 4 Hartmut Goldschmidt, 5 Andrzej Jakubowiak, 6 Jesus San-Miguel, 7 Mihaela Obreja, 8 Julie Blaedel, 8 Meletios A. Dimopoulos 9 on behalf of the ASPIRE Investigators 1 Mayo Clinic, Scottsdale, AZ, USA; 2 John Theurer Cancer Center, Hackensack University Medical Center, Hackensack, NJ, USA; 3 Wilheminen Cancer Research Institute, Vienna, Austria; 4 CHRU Lille Hôpital Claude Huriez, Lille, France; 5 University of Heidelberg, Germany; 6 University of Chicago, IL, USA; 7 Clinica Universidad de Navarra–Centro de Investigación Médica Aplicada, IDISNA, CIBERONC, Pamplona, Spain; 8 Amgen Inc. , Thousand Oaks, CA, USA; 9 National and Kapodistrian University of Athens School of Medicine, Athens, Greece SC-CH-CARFILZOMI-00115

Background: Phase 3 ASPIRE Trial • The phase 3 ENDEAVOR trial (carfilzomib and dexamethasone vs bortezomib and dexamethasone) was the first to describe a statistically significant prolongation of OS using a novel therapy vs standard of care 1 • As previously reported the addition of carfilzomib to lenalidomide and dexamethasone resulted in more frequent and deeper responses when compared with Rd alone which translated into longer progression-free survival (PFS), for patients with relapsed or refractory multiple myeloma (RRMM)2 – ≥ Complete response : 31. 8% vs 9. 3% – PFS: median 26. 3 months KRd vs 17. 6 months Rd; HR, 0. 69; 2 -sided P=0. 0001 – Improved patient reported health-related quality of life 3 • Here we report the pre-specified final OS data and updated safety results from the ASPIRE study 1 Dimopoulos MA, et al. Lancet Oncol. 2017; 18: 1327 -1337. Engl J Med. 2015; 372: 142 -152. 3 Stewart AK, et al. J Clin Oncol. 2016; 34: 3921 -3930. 2 Stewart AK, et al. N

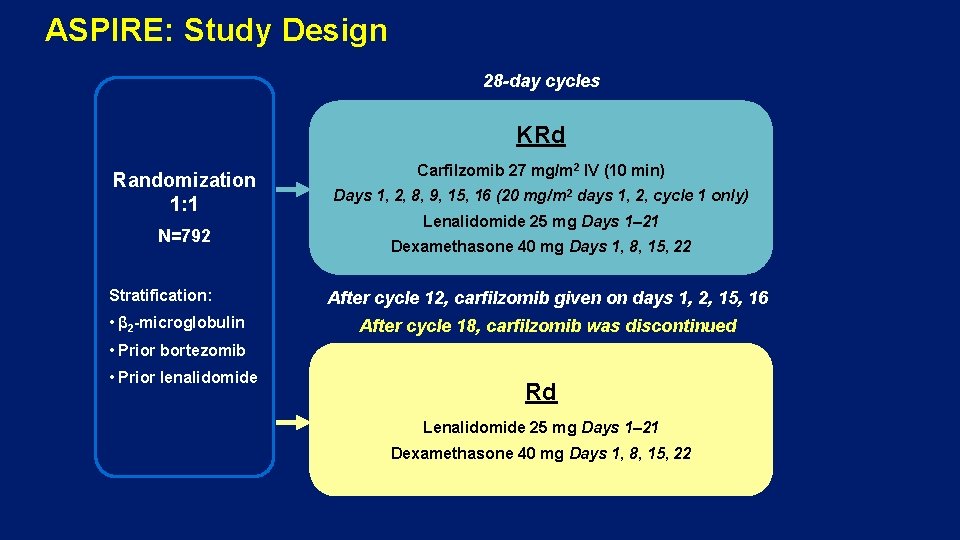
ASPIRE: Study Design 28 -day cycles KRd Randomization 1: 1 N=792 Stratification: • β 2 -microglobulin Carfilzomib 27 mg/m 2 IV (10 min) Days 1, 2, 8, 9, 15, 16 (20 mg/m 2 days 1, 2, cycle 1 only) Lenalidomide 25 mg Days 1– 21 Dexamethasone 40 mg Days 1, 8, 15, 22 After cycle 12, carfilzomib given on days 1, 2, 15, 16 After cycle 18, carfilzomib was discontinued • Prior bortezomib • Prior lenalidomide Rd Lenalidomide 25 mg Days 1– 21 Dexamethasone 40 mg Days 1, 8, 15, 22

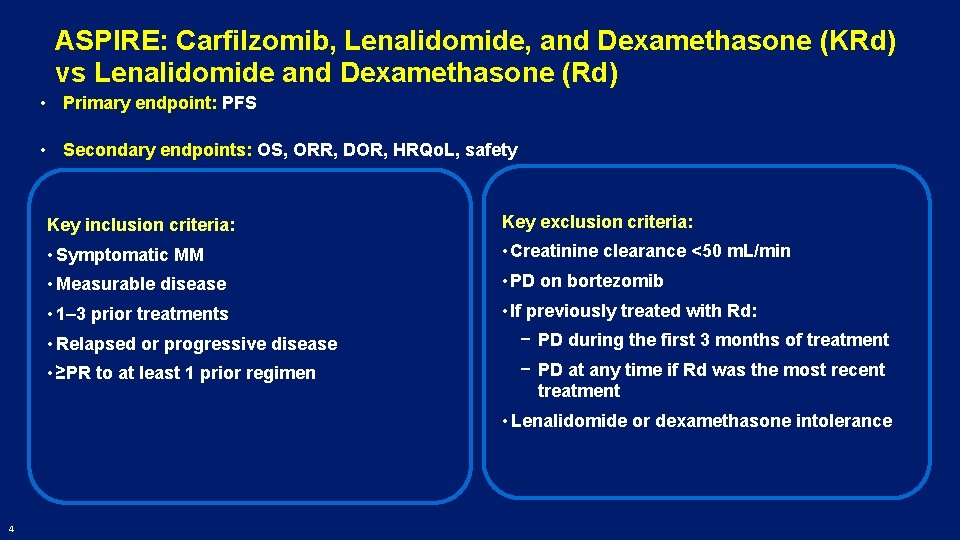
ASPIRE: Carfilzomib, Lenalidomide, and Dexamethasone (KRd) vs Lenalidomide and Dexamethasone (Rd) • Primary endpoint: PFS • Secondary endpoints: OS, ORR, DOR, HRQo. L, safety Key inclusion criteria: Key exclusion criteria: • Symptomatic MM • Creatinine clearance <50 m. L/min • Measurable disease • PD on bortezomib • 1– 3 prior treatments • If previously treated with Rd: • Relapsed or progressive disease − PD during the first 3 months of treatment • ≥PR to at least 1 prior regimen − PD at any time if Rd was the most recent treatment • Lenalidomide or dexamethasone intolerance 4

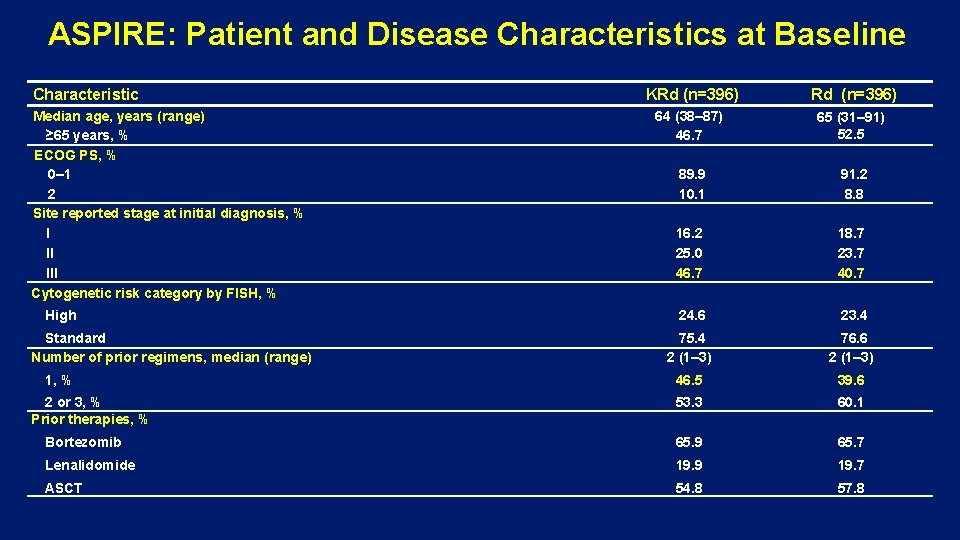
ASPIRE: Patient and Disease Characteristics at Baseline Characteristic KRd (n=396) 64 (38– 87) 46. 7 65 (31– 91) 52. 5 89. 9 10. 1 91. 2 8. 8 16. 2 25. 0 46. 7 18. 7 23. 7 40. 7 24. 6 23. 4 75. 4 2 (1– 3) 76. 6 2 (1– 3) 46. 5 39. 6 53. 3 60. 1 Bortezomib 65. 9 65. 7 Lenalidomide 19. 9 19. 7 ASCT 54. 8 57. 8 Median age, years (range) ≥ 65 years, % ECOG PS, % 0– 1 2 Site reported stage at initial diagnosis, % I II III Cytogenetic risk category by FISH, % High Standard Number of prior regimens, median (range) 1, % 2 or 3, % Prior therapies, %

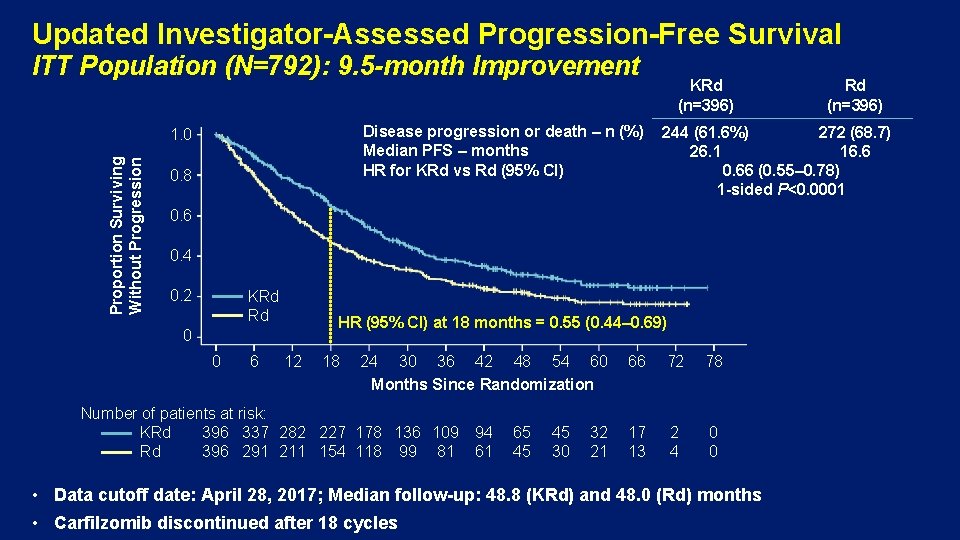
Updated Investigator-Assessed Progression-Free Survival ITT Population (N=792): 9. 5 -month Improvement Disease progression or death – n (%) Median PFS – months HR for KRd vs Rd (95% CI) Proportion Surviving Without Progression 1. 0 0. 8 KRd (n=396) 244 (61. 6%) 272 (68. 7) 26. 1 16. 6 0. 66 (0. 55– 0. 78) 1 -sided P<0. 0001 0. 6 0. 4 0. 2 KRd Rd HR (95% CI) at 18 months = 0. 55 (0. 44– 0. 69) 0 0 6 12 18 24 30 36 42 48 54 60 Months Since Randomization Number of patients at risk: KRd 396 337 282 227 178 136 109 Rd 396 291 211 154 118 99 81 94 61 65 45 45 30 32 21 Rd (n=396) 66 72 78 17 13 2 4 0 0 • Data cutoff date: April 28, 2017; Median follow-up: 48. 8 (KRd) and 48. 0 (Rd) months • Carfilzomib discontinued after 18 cycles

ASPIRE: Overall Survival Analysis • The preplanned final analysis for OS was to be performed after 510 deaths occurred, which would provide 85% power to detect, with a 1 -sided significance level of 0. 025, a 23. 5% reduction in the risk of death for KRd vs Rd – Significance level was determined by the O’Brien-Fleming type alpha spending function based on the actual number of events (1 -sided alpha=0. 023) • Data cutoff was April 28, 2017 with a median OS follow up of ~67 months

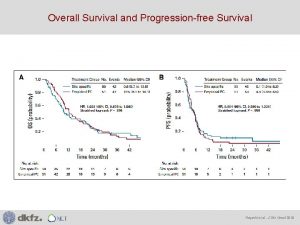
Overall Survival: 7. 9 -month Median Improvement KRd (n=396) Proportion Surviving 1. 0 Death – n (%) Median OS – months HR for KRd vs Rd (95% CI) 0. 8 Rd (n=396) 246 (62. 1) 267 (67. 4) 48. 3 40. 4 0. 79 (0. 67– 0. 95) 1 -sided P=0. 0045 0. 6 0. 4 0. 2 KRd Rd Events at 18 months: KRd, 71 (17. 9%); Rd, 97 (24. 5%) HR (95% CI) = 0. 69 (0. 51– 0. 93) 0 0 6 12 18 24 30 36 42 48 54 60 66 72 78 Months Since Randomization Number of patients at risk: KRd Rd 396 369 356 343 316 281 282 243 259 220 232 199 211 176 190 149 166 133 149 113 88 69 22 20 0 3

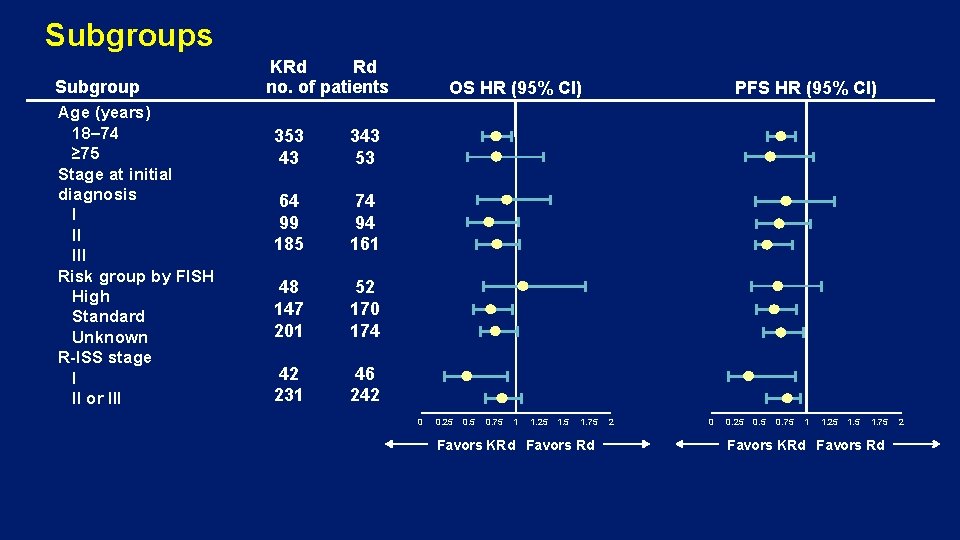
Subgroups Subgroup Age (years) 18– 74 ≥ 75 Stage at initial diagnosis I II III Risk group by FISH High Standard Unknown R-ISS stage I II or III KRd Rd no. of patients 353 43 343 53 64 99 185 74 94 161 48 147 201 52 170 174 42 231 46 242 OS HR (95% CI) 0 0. 25 0. 75 1 1. 25 1. 75 Favors KRd Favors Rd PFS HR (95% CI) 2 0 0. 25 0. 75 1 1. 25 1. 75 Favors KRd Favors Rd 2

Subgroups (Genetic Risk) • High risk was defined as t(4; 14), t(14; 16), or deletion 17 p in ≥ 60% of plasma cells • The proportion of patients with unknown cytogenetics was 50. 8% (KRd) and 43. 9% (Rd) • Only 48 (KRd) and 52 patients (Rd) were determined as high risk - ORRs in the high-risk KRd group were higher than those in the Rd: 79. 2% vs 59. 6%1 - For high-risk genetic patients, treatment with KRd resulted in a 9 -month improvement in median PFS vs Rd (HR, 0. 70)1 • Fewer high-risk cytogenetics patients received subsequent therapy (KRd 39. 6% vs Rd 57. 7%) 1 Avet-Loiseau H, et al. Blood. 2016; 128: 1174 -1180.

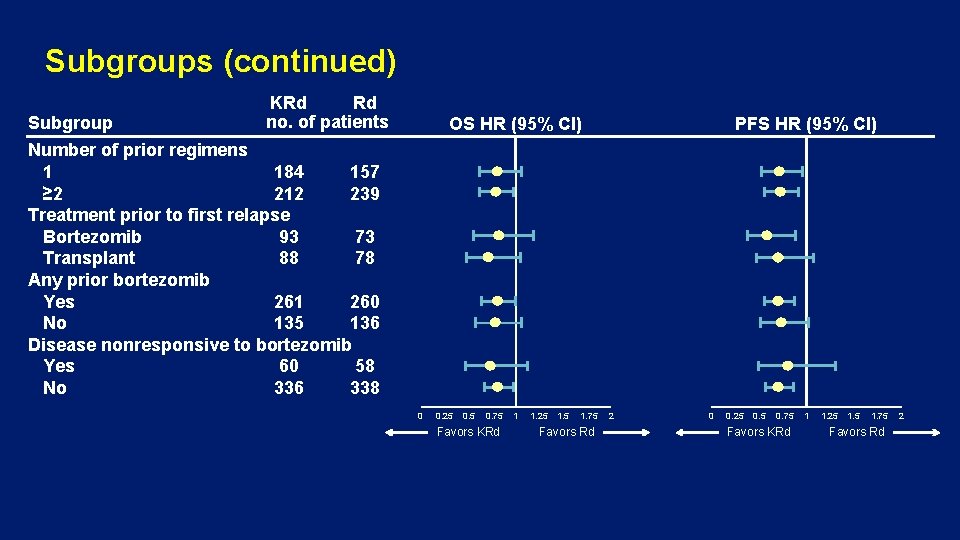
Subgroups (continued) Subgroup KRd Rd no. of patients OS HR (95% CI) PFS HR (95% CI) Number of prior regimens 1 184 157 ≥ 2 212 239 Treatment prior to first relapse Bortezomib 93 73 Transplant 88 78 Any prior bortezomib Yes 261 260 No 135 136 Disease nonresponsive to bortezomib Yes 60 58 No 336 338 0 0. 25 0. 75 Favors KRd 1 1. 25 1. 75 Favors Rd 2

Subgroups (continued) • Median OS was 11. 4 months longer for KRd vs Rd for patients at first relapse (47. 3 vs 35. 9 months) • Median OS was 12 months longer with KRd vs Rd for patients who received bortezomib prior to first relapse (45. 9 vs 33. 9 months) • Median OS was 18. 6 months longer for patients receiving transplant prior to first relapse (57. 2 vs 38. 6 months)

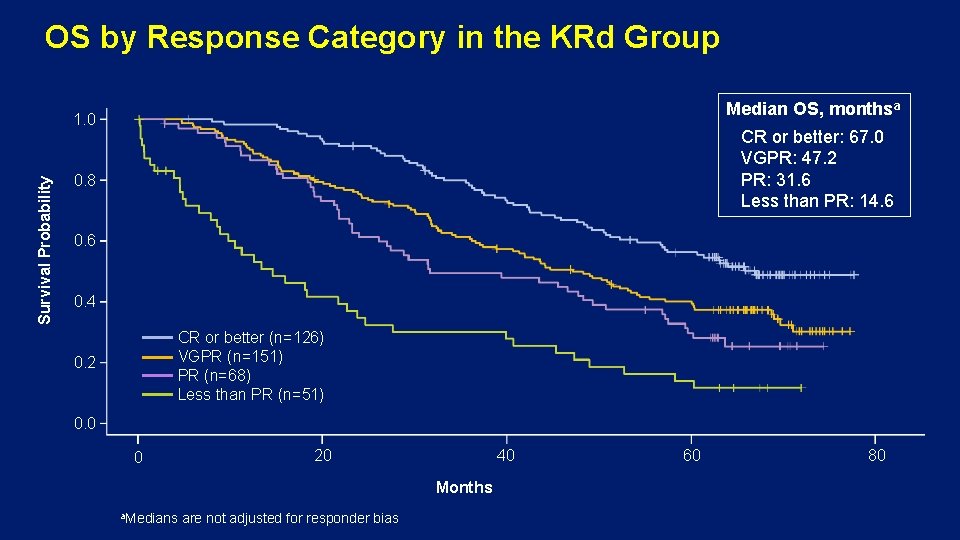
OS by Response Category in the KRd Group Median OS, monthsa Survival Probability 1. 0 CR or better: 67. 0 VGPR: 47. 2 PR: 31. 6 Less than PR: 14. 6 0. 8 0. 6 0. 4 CR or better (n=126) VGPR (n=151) PR (n=68) Less than PR (n=51) 0. 2 0. 0 0 20 40 Months a. Medians are not adjusted for responder bias 60 80

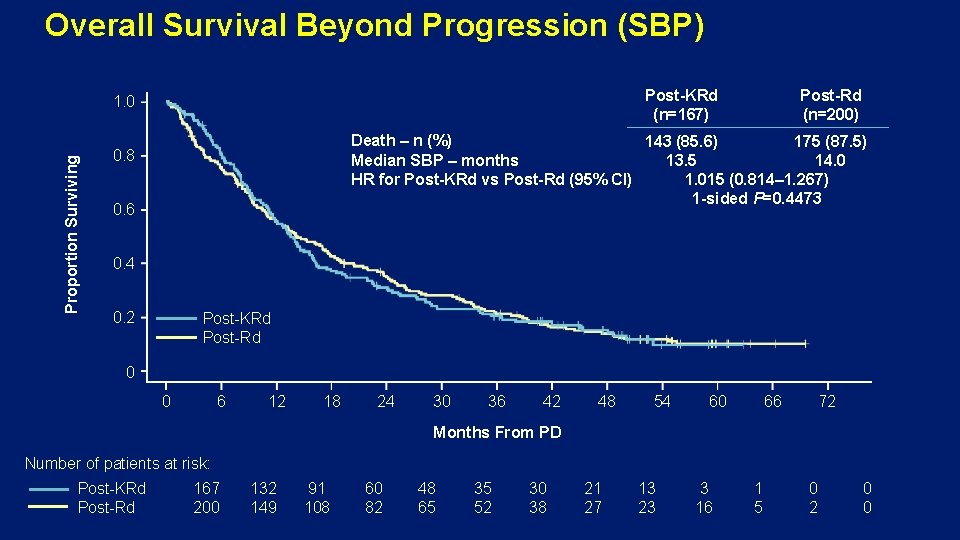
Overall Survival Beyond Progression (SBP) Post-KRd (n=167) Proportion Surviving 1. 0 Post-Rd (n=200) Death – n (%) 143 (85. 6) 175 (87. 5) Median SBP – months 13. 5 14. 0 HR for Post-KRd vs Post-Rd (95% CI) 1. 015 (0. 814– 1. 267) 1 -sided P=0. 4473 0. 8 0. 6 0. 4 0. 2 Post-KRd Post-Rd 0 0 6 12 18 24 30 36 42 48 54 60 66 72 Months From PD Number of patients at risk: Post-KRd Post-Rd 167 200 132 149 91 108 60 82 48 65 35 52 30 38 21 27 13 23 3 16 1 5 0 2 0 0

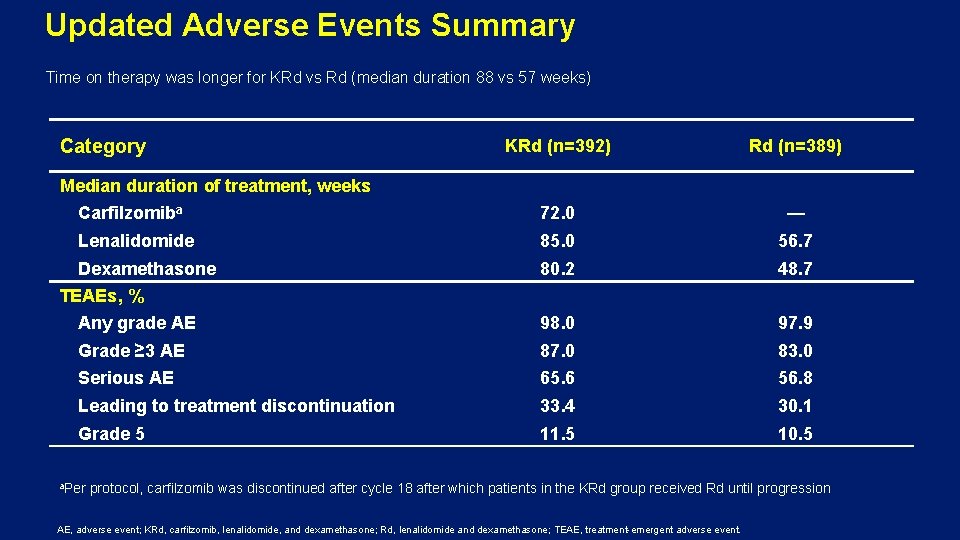
Updated Adverse Events Summary Time on therapy was longer for KRd vs Rd (median duration 88 vs 57 weeks) Category KRd (n=392) Rd (n=389) Carfilzomiba 72. 0 — Lenalidomide 85. 0 56. 7 Dexamethasone 80. 2 48. 7 Any grade AE 98. 0 97. 9 Grade ≥ 3 AE 87. 0 83. 0 Serious AE 65. 6 56. 8 Leading to treatment discontinuation 33. 4 30. 1 Grade 5 11. 5 10. 5 Median duration of treatment, weeks TEAEs, % a. Per protocol, carfilzomib was discontinued after cycle 18 after which patients in the KRd group received Rd until progression AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; TEAE, treatment-emergent adverse event.

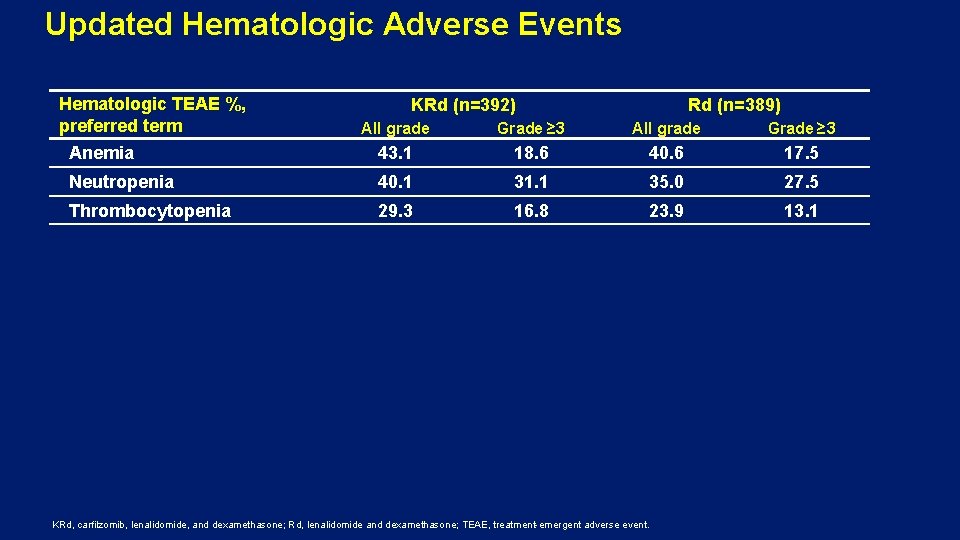
Updated Hematologic Adverse Events Hematologic TEAE %, preferred term KRd (n=392) Rd (n=389) All grade Grade ≥ 3 Anemia 43. 1 18. 6 40. 6 17. 5 Neutropenia 40. 1 31. 1 35. 0 27. 5 Thrombocytopenia 29. 3 16. 8 23. 9 13. 1 KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; TEAE, treatment-emergent adverse event.

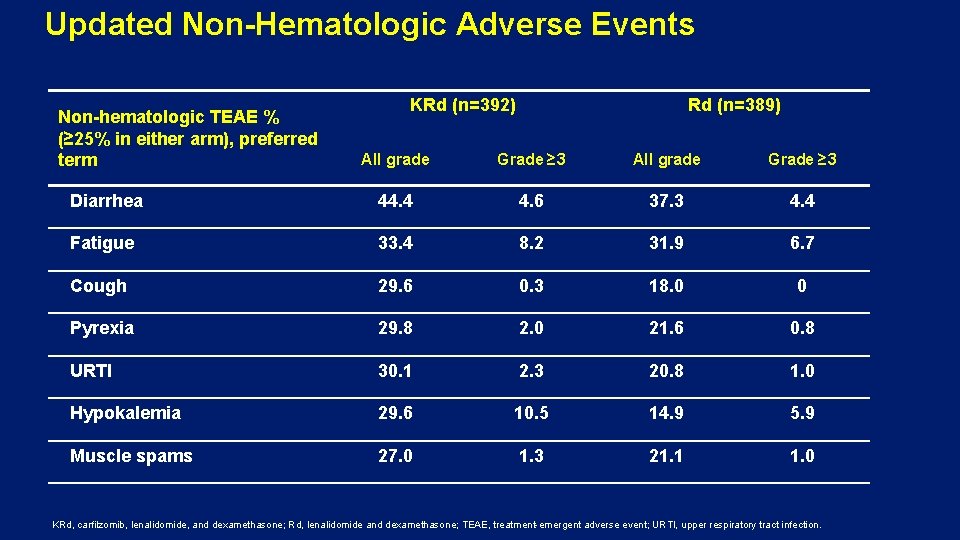
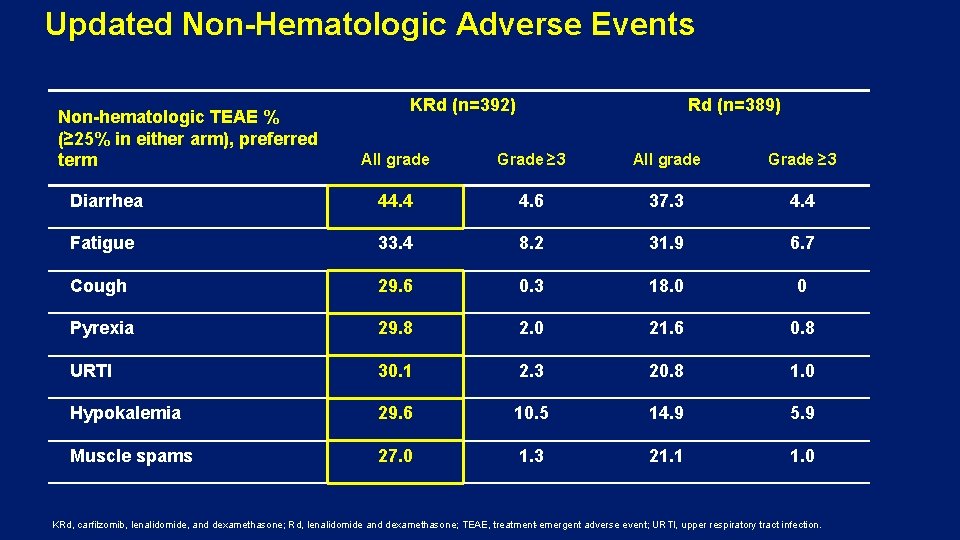
Updated Non-Hematologic Adverse Events Non-hematologic TEAE % (≥ 25% in either arm), preferred term KRd (n=392) Rd (n=389) All grade Grade ≥ 3 Diarrhea 44. 4 4. 6 37. 3 4. 4 Fatigue 33. 4 8. 2 31. 9 6. 7 Cough 29. 6 0. 3 18. 0 0 Pyrexia 29. 8 2. 0 21. 6 0. 8 URTI 30. 1 2. 3 20. 8 1. 0 Hypokalemia 29. 6 10. 5 14. 9 5. 9 Muscle spams 27. 0 1. 3 21. 1 1. 0 KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; TEAE, treatment-emergent adverse event; URTI, upper respiratory tract infection.

Updated Non-Hematologic Adverse Events Non-hematologic TEAE % (≥ 25% in either arm), preferred term KRd (n=392) Rd (n=389) All grade Grade ≥ 3 Diarrhea 44. 4 4. 6 37. 3 4. 4 Fatigue 33. 4 8. 2 31. 9 6. 7 Cough 29. 6 0. 3 18. 0 0 Pyrexia 29. 8 2. 0 21. 6 0. 8 URTI 30. 1 2. 3 20. 8 1. 0 Hypokalemia 29. 6 10. 5 14. 9 5. 9 Muscle spams 27. 0 1. 3 21. 1 1. 0 KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; TEAE, treatment-emergent adverse event; URTI, upper respiratory tract infection.

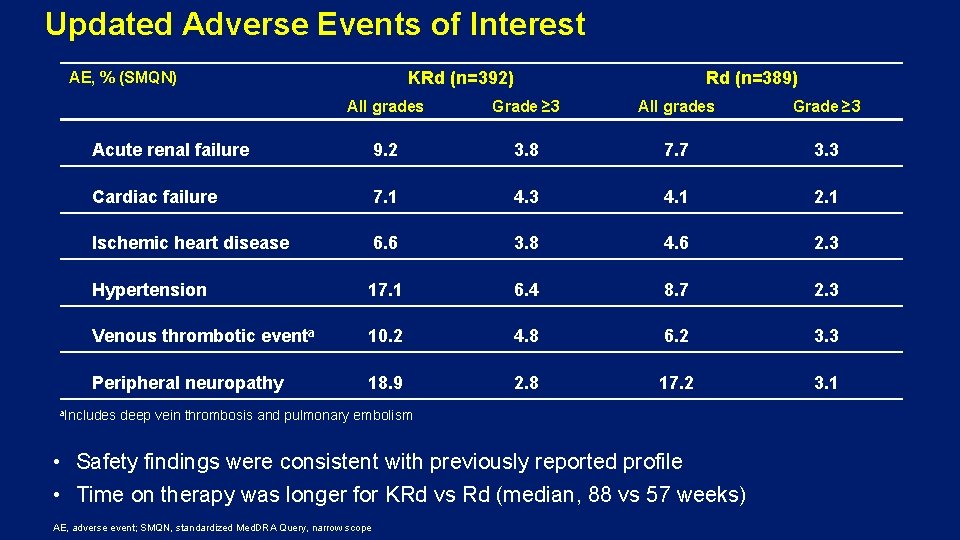
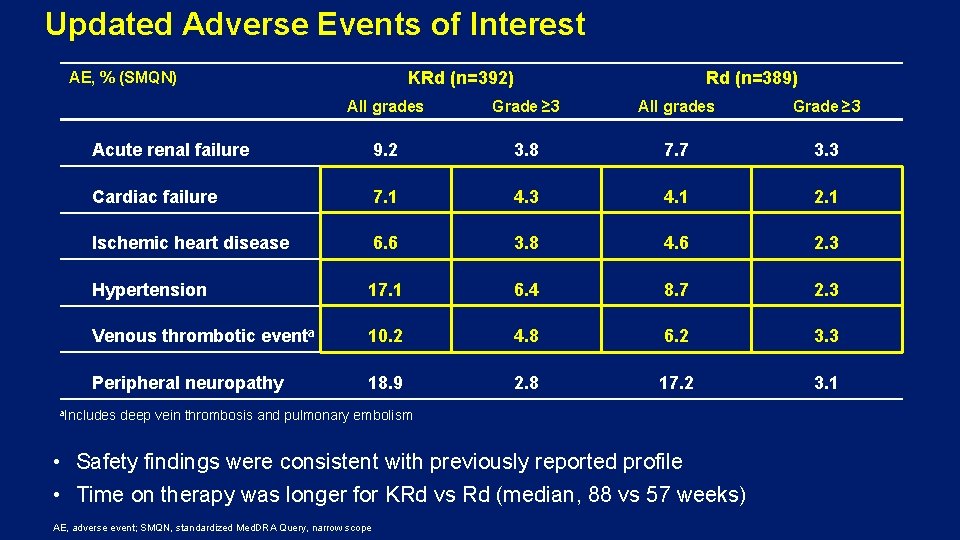
Updated Adverse Events of Interest KRd (n=392) AE, % (SMQN) Rd (n=389) All grades Grade ≥ 3 Acute renal failure 9. 2 3. 8 7. 7 3. 3 Cardiac failure 7. 1 4. 3 4. 1 2. 1 Ischemic heart disease 6. 6 3. 8 4. 6 2. 3 Hypertension 17. 1 6. 4 8. 7 2. 3 Venous thrombotic eventa 10. 2 4. 8 6. 2 3. 3 Peripheral neuropathy 18. 9 2. 8 17. 2 3. 1 a. Includes deep vein thrombosis and pulmonary embolism • Safety findings were consistent with previously reported profile • Time on therapy was longer for KRd vs Rd (median, 88 vs 57 weeks) AE, adverse event; SMQN, standardized Med. DRA Query, narrow scope

Updated Adverse Events of Interest KRd (n=392) AE, % (SMQN) Rd (n=389) All grades Grade ≥ 3 Acute renal failure 9. 2 3. 8 7. 7 3. 3 Cardiac failure 7. 1 4. 3 4. 1 2. 1 Ischemic heart disease 6. 6 3. 8 4. 6 2. 3 Hypertension 17. 1 6. 4 8. 7 2. 3 Venous thrombotic eventa 10. 2 4. 8 6. 2 3. 3 Peripheral neuropathy 18. 9 2. 8 17. 2 3. 1 a. Includes deep vein thrombosis and pulmonary embolism • Safety findings were consistent with previously reported profile • Time on therapy was longer for KRd vs Rd (median, 88 vs 57 weeks) AE, adverse event; SMQN, standardized Med. DRA Query, narrow scope

Overall Survival Conclusions • KRd demonstrated a statistically significant and clinically meaningful reduction in the risk of death vs Rd, improving median OS by 7. 9 months (48. 3 vs 40. 4 months; HR, 0. 79, P=0. 0045) • The KRd efficacy advantage is most pronounced at first relapse, with an 11 month improvement in median OS (47. 3 vs 35. 9 months; HR, 0. 81) • Treatment with KRd did not compromise OS after relapse

Summary • The addition of carfilzomib to Rd resulted in a higher ORR, a tripling of the complete response rate, significant and clinically relevant prolongation of both PFS 1 and OS and improved HRQo. L 2 • Safety is consistent with previous findings and no new safety signals were observed for KRd after extended follow-up • ASPIRE is the second phase 3 trial in RRMM to demonstrate a statistically significant prolongation of OS with a carfilzomib-based regimen 3 • KRd is an excellent treatment option proven to extend survival in RRMM, particularly at first relapse 1 Stewart AK, et al. N Engl J Med. 2015; 372: 142 -152. Clin Oncol. 2016; 34: 3921 -3930. 3 Dimopoulos MA, et al. Lancet Oncol. 2017; 18: 1327 -1337. 2 Stewart AK, et al. J

Conflict of Interest Disclosure • Acknowledgments This study was supported by Onyx Pharmaceuticals, Inc. , an Amgen Inc. subsidiary. Medical writing assistance was provided by Blue. Momentum, an Ashfield company, part of UDG Healthcare plc, and funded by Amgen Inc. • Financial Disclosures Stewart: Amgen, Celgene, Bristol-Myers Squibb, Janssen, Takeda, Seattle Genetics: Consultancy, Research Funding, Honoraria. Siegel: Celgene, Takeda, Amgen Inc, Novartis and BMS: Honoraria, Speakers Bureau. Ludwig: Takeda and Amgen: Consultancy, Research Funding, Speakers Bureau; Cilag-Janssen: Consultancy, Speakers Bureau; Celgene and BMS: Speakers Bureau. Facon: Amgen, Celgene: Speakers Bureau. Goldschmidt: Amgen, Celgene, Chugai, Janssen, Novartis, and BMS: Consultancy, Research Funding, Honoraria. Jakubowiak: University of Chicago: Employment; Amgen Inc. , BMS, Celgene, Janssen, Karypharm, Millennium-Takeda, Sanofi, Skyline. DX: Consultancy, Research Funding, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. San Miguel: Amgen, BMS, Celgene, Janssen, MSD, Novartis, Takeda, Sanofi, Roche: Other Consulting or advisory role. Obreja: Amgen: Employment, Equity Ownership. Blaedel: Amgen: Employment, Equity Ownership. Dimopoulos: Amgen, Celgene, Janssen, Novartis, and Takeda: Consultancy. SC-CH-CARFILZOMI-00115
 Waldenstrom's disease
Waldenstrom's disease Mayo clinic multiple myeloma
Mayo clinic multiple myeloma Smoldering lymphoma
Smoldering lymphoma üig
üig Smoldering multiple myeloma
Smoldering multiple myeloma Smoldering multiple myeloma
Smoldering multiple myeloma Waldenstrom macroglobulinemia vs multiple myeloma
Waldenstrom macroglobulinemia vs multiple myeloma Mielma
Mielma Kpd myeloma
Kpd myeloma Median overall survival
Median overall survival Overall survival icon
Overall survival icon State of survival survival of the fittest tweak
State of survival survival of the fittest tweak State of survival survival of the fittest stages
State of survival survival of the fittest stages Daratumumab macmillan
Daratumumab macmillan European myeloma network
European myeloma network Myeloma
Myeloma Anita waldmann
Anita waldmann Myeloma uk forum
Myeloma uk forum Example of mimd
Example of mimd Multiple probe vs multiple baseline
Multiple probe vs multiple baseline Edward oxford
Edward oxford Ethical issues in treating lgbt patients
Ethical issues in treating lgbt patients Module 70 introduction to therapy
Module 70 introduction to therapy Management of patients with neurologic trauma
Management of patients with neurologic trauma