OpioidSparing Assessing opioid use and opioid outcomes in


















- Slides: 28

Opioid(-)Sparing: Assessing opioid use and opioid outcomes in clinical trials Brett R. Stacey, MD Medical Director, UW Center for Pain Relief Professor, Anesthesiology & Pain Medicine

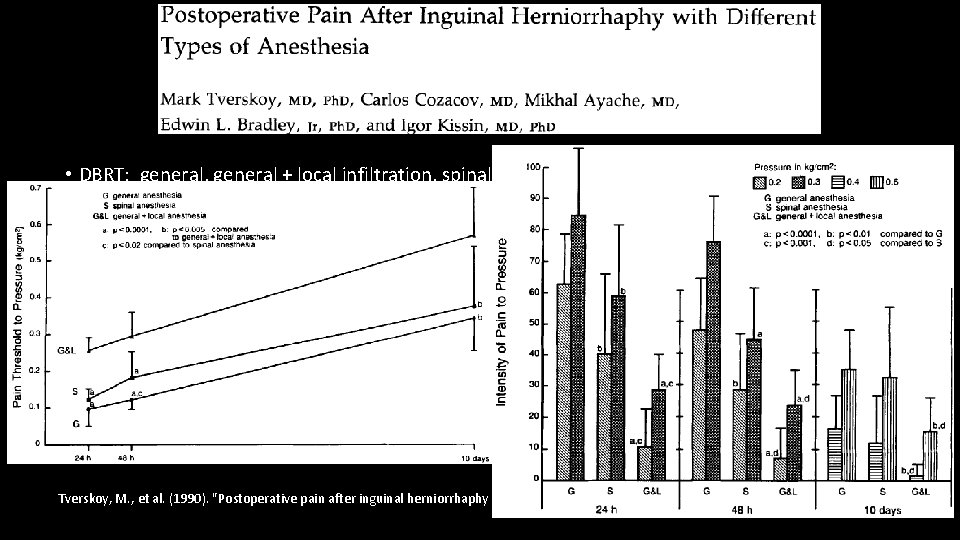
• DBRT: general, general + local infiltration, spinal • The main focus of the paper was pain at rest, pain with pressure, pain with movement up to 10 days Tverskoy, M. , et al. (1990). "Postoperative pain after inguinal herniorrhaphy with different types of anesthesia. " Anesthesia & Analgesia 70(1): 29 -35.

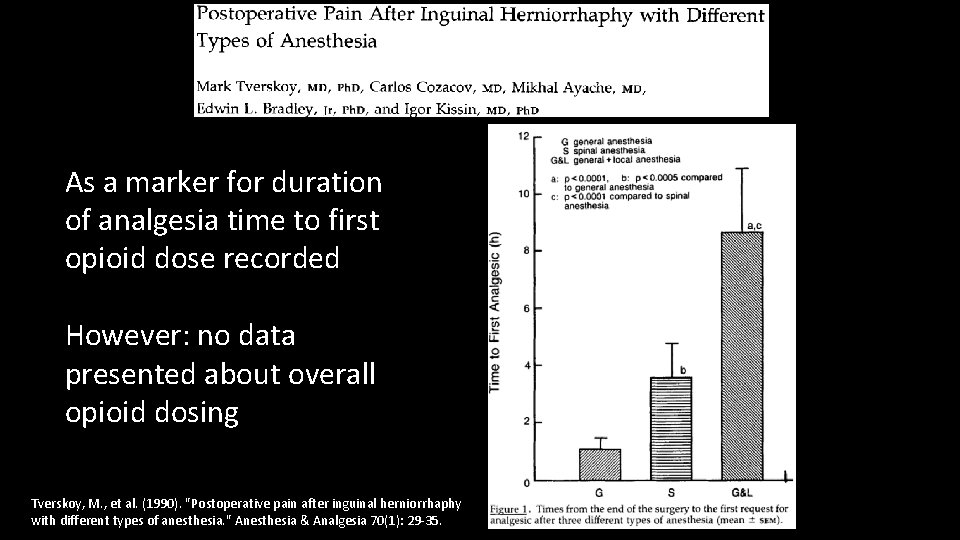
As a marker for duration of analgesia time to first opioid dose recorded However: no data presented about overall opioid dosing Tverskoy, M. , et al. (1990). "Postoperative pain after inguinal herniorrhaphy with different types of anesthesia. " Anesthesia & Analgesia 70(1): 29 -35.

It May Be Obvious • Opioid reduction often not the focus of the study = the data can be buried • Opioid reduction is meaningless if pain is worse • For acute pain = no standard for how to assess opioid use, dose reduction, or opioid specific outcomes, but there are many studies reporting “opioid-sparing” • For chronic pain = even less consistent data

Lack of data is no barrier to lots of discussion

How to assess opioid use in clinical trials? • Time to first dose/ Use of rescue • Dose prescribed • # of pills/doses prescribed per event or time period • # of pills/doses taken • MME/MED or cumulative/daily dose • Duration of opioid therapy • Opioid refills • Dose escalation • Dose of zero • Pain Outcomes (see other IMMPACT info): • Pain rating = universal in some form • Function = variable • Satisfaction = variable • Pain, function, side effects, satisfaction: • Not worsened • Unchanged or improved • Multiple assessment options

Time to first dose/ Use of rescue • Commonly used in Acute Pain trials to assess an intervention’s initial effect or duration of effect. • With less intense pain stimulus or more effective intervention rescue may be avoided. • What is clinically significant? • Is it meaningful as a stand alone assessment? • How does it translate to clinical practice? • Does it lead to decreased overall opioid dosing?

MME/MED • MME = morphine milligram equivalents • MED = morphine equivalent dose • Risk for opioid-related serious harms are dose-dependent • Both Acute and Chronic pain studies • In acute pain studies: often 24 -48 hours only • Clinical utility as well • Prominent in public policy and guidelines • Reported per time period (commonly daily) or totaled over the study period

Prescribed Dose/# of Pills • Opioids often prescribed/consumed in defined increments either scheduled or “as needed” • Most prescription opioid consumption for both acute and chronic pain occurs outside of the supervised medical setting • Higher doses = more risk, more adverse effects • More pills = more available for diversion/misuse

Acute Pain

Acute Pain: Opioid-Sparing Review • Review of studies demonstrating “opioid-sparing: ” adjuvant medications, regional anesthesia, nerve blocks • Reduced opioid consumption first 24 - 48 hours = most commonly reported opioid-sparing effect “While individual pieces of optimal postoperative pain management plans have been studied, long-term outcome data are lacking, as well as data regarding rebound pain…. there is much work to be done. Kumar, K. , et al. (2017). "A Review of Opioid-Sparing Modalities in Perioperative Pain Management: Methods to Decrease Opioid Use Postoperatively. " Anesthesia & Analgesia 125(5): 1749 -1760.

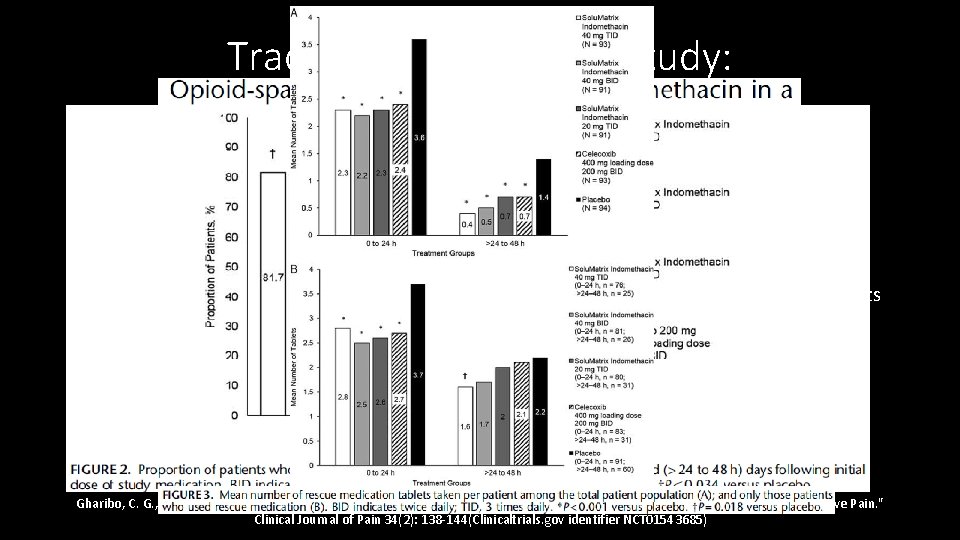
Traditional PCRT Drug Study: • Phase 3, multicenter DBPCRT Solu. Matrix indomethacin, 462 subjects, bunionectomy • All subjects: popliteal sciatic block + 1 st metatarsal block, continued till POD #1, study medication given on POD #1 for 48 hours • Randomized to 3 different doses of Solu. Matrix indomethacin, celecoxib, or placebo. • Outcome measures: Pain Intensity Difference treatment groups vs Placebo, proportion of patients who used rescue (hydrocodone/apap), time to first use of rescue, number of tablets • Results: active treatment resulted in reductions that were most pronounced after 24 hours • Notes: • Given the delay, starting early may have led to greater separation between groups • What happens after the 48 hour study period? Gharibo, C. G. , et al. (2018). "Opioid-sparing Effects of Solu. Matrix Indomethacin in a Phase 3 Study in Patients With Acute Postoperative Pain. " Clinical Journal of Pain 34(2): 138 -144(Clinicaltrials. gov identifier NCT 01543685)

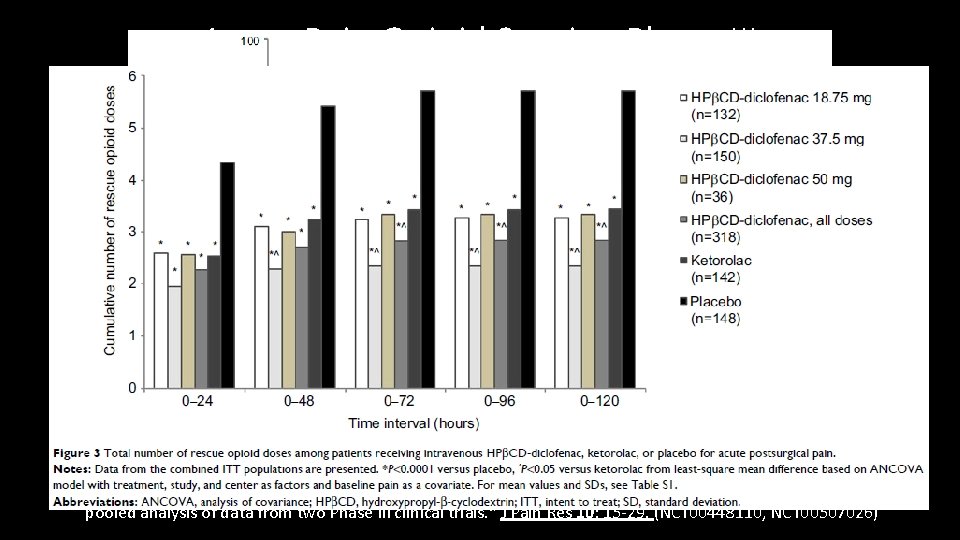
Acute Pain Opioid Sparing Phase III • Two DBPCRTs abdominal/pelvic or orthopedic surgery, hydroxypropyl-β-cyclodextrin (HPβCD)-diclofenac, ketorolac, or placebo, 608 subjects. • Intervention: IV NSAID or placebo. Dose adjusted by non-pain factors (wt, risks, age) • Opioid assessments: IV Morphine bolus. Proportion of patient requiring opioids, cumulative opioid dose and number of doses over 5 days. • Results: treatment groups were less likely to use opioids, used fewer doses, fewer mg • Notes: not the primary outcomes of the studies, this is a combined secondary paper focused on opioids with varied patient population Gan, T. J. , et al. (2017). "Postoperative opioid sparing with injectable hydroxypropyl-beta-cyclodextrin-diclofenac: pooled analysis of data from two Phase III clinical trials. " J Pain Res 10: 15 -29. (NCT 00448110, NCT 00507026)

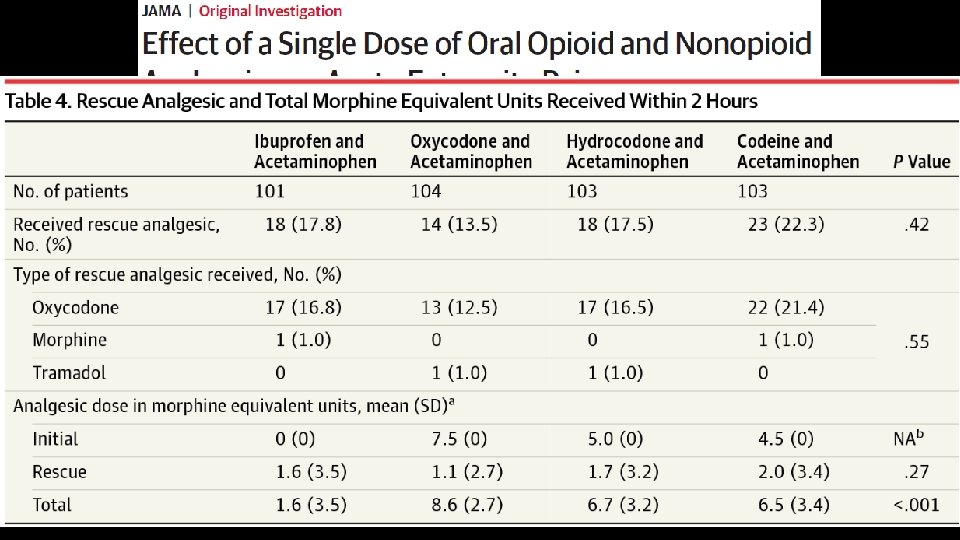
• 416 patients with acute moderate to severe extremity pain, randomized • Outcomes assessed after 2 hours (remember, it is an ED study) Chang, A. K. , et al. (2017). "Effect of a Single Dose of Oral Opioid and Nonopioid Analgesics on Acute Extremity Pain in the Emergency Department: A Randomized Clinical Trial. " JAMA 318(17): 1661 -1667.

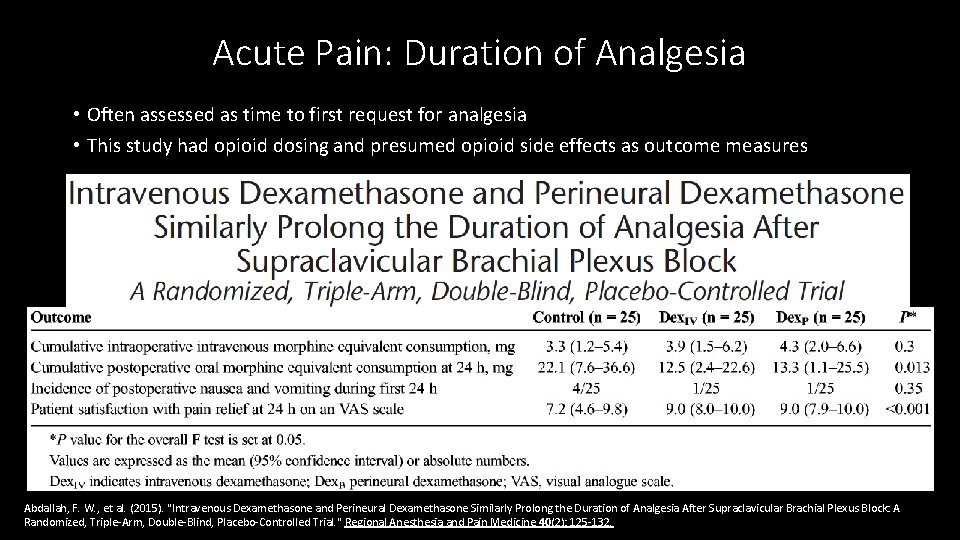
Acute Pain: Duration of Analgesia • Often assessed as time to first request for analgesia • This study had opioid dosing and presumed opioid side effects as outcome measures Abdallah, F. W. , et al. (2015). "Intravenous Dexamethasone and Perineural Dexamethasone Similarly Prolong the Duration of Analgesia After Supraclavicular Brachial Plexus Block: A Randomized, Triple-Arm, Double-Blind, Placebo-Controlled Trial. " Regional Anesthesia and Pain Medicine 40(2): 125 -132.

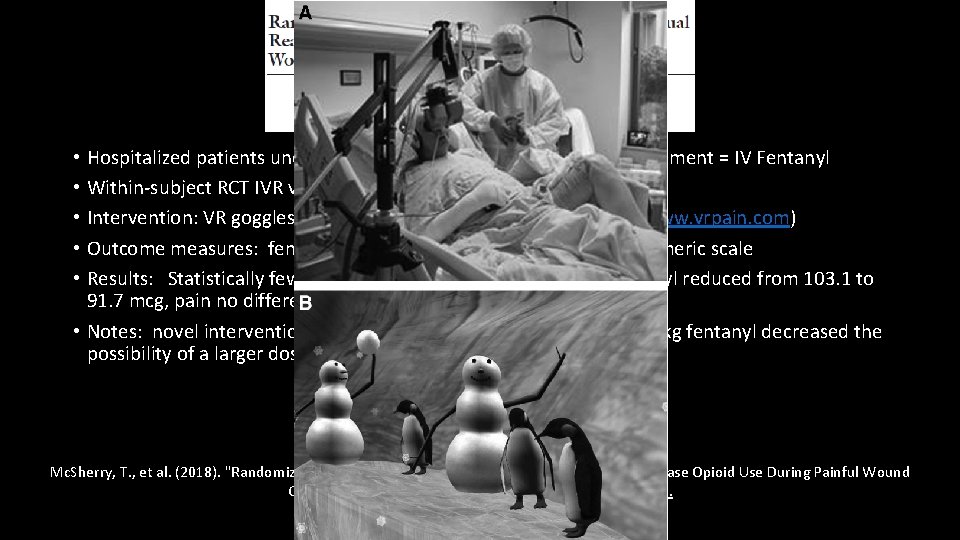
• Hospitalized patients undergoing painful wound care, standard treatment = IV Fentanyl • Within-subject RCT IVR vs no IVR; 18 subjects • Intervention: VR goggles, headset, use of mouse in Snow. World (www. vrpain. com) • Outcome measures: fentanyl total dose, # of doses, 0 -10 verbal numeric scale • Results: Statistically fewer requests for opioid, total dose of fentanyl reduced from 103. 1 to 91. 7 mcg, pain no different. • Notes: novel intervention. Standardized pretreatment with 1 mcg/kg fentanyl decreased the possibility of a larger dose reduction. Mc. Sherry, T. , et al. (2018). "Randomized, Crossover Study of Immersive Virtual Reality to Decrease Opioid Use During Painful Wound Care Procedures in Adults. " J Burn Care Res 39(2): 278 -285.

ERAS • Pre-emptive analgesia, preventive analgesia: a focus on delaying/minimizing pain, often with one single intervention • Multimodal analgesia: mainly anesthesia interventions, analgesia in the title • Enhanced Recovery After Surgery: (ERAS): many interventions, systematic multifaceted team approach spanning periop period, multiple objectives, improved pain control not the sole focus. • One of the commonly reported benefits of ERAS protocols is reduced reliance on opioids • How that is reported is variable: • Meta-analysis #1: Lau & Chamberlain 2016: 42 RCTs reviewed, mainly reported LOS, readmission, costs, complications, GI function, etc. DID NOT REPORT OPIOID DOSING/OPIOID REDUCTION • Review #2: Wick et al 2017: prominent focus on opioids, overview, specific measures not reported Lau, C. S. and R. S. Chamberlain (2017). "Enhanced Recovery After Surgery Programs Improve Patient Outcomes and Recovery: A Meta-analysis. " World J Surg 41(4): 899 -913. Wick, E. C. , et al. (2017). "Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. " JAMA Surg 152(7): 691 -697.

Prescribed opioids for surgery • Across multiple procedures: 42 -71% opioids unused and typically available for unmonitored use • Persistent opioid use just as likely after minor surgery • Prescribing less • Patients just as satisfied • Don’t have more pain • Use fewer pills (lower doses) for shorter duration • Not more likely to get refills 1. 2. 3. 4. 5. Bicket, M. C. , et al. (2017). "Prescription Opioid Analgesics Commonly Unused After Surgery: A Systematic Review. " JAMA Surg. Brummett, C. M. , et al. (2017). "New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. " JAMA Surg 152(6): e 170504. Sekhri, S. , et al. (2017). "Probability of Opioid Prescription Refilling After Surgery: Does Initial Prescription Dose Matter? " Ann Surg. Waljee, J. F. , et al. (2017). "Iatrogenic Opioid Dependence in the United States: Are Surgeons the Gatekeepers? " Ann Surg 265(4): 728 -730. Lee, J. S. , et al. (2017). "Postoperative Opioid Prescribing and the Pain Scores on Hospital Consumer Assessment of Healthcare Providers and Systems Survey. " Jama 317(19): 2013 -2015.

Chronic Pain

Chronic Pain Opioid Reduction Cochrane Review, 2017 • Examining interventions to reduce prescribed opioid use in chronic pain • Heterogeneous populations, interventions. and outcomes. 5 studies identified • They decided what a responder was: • “at least a 50% reduction in opioid consumption” • Or: Complete opioid withdrawal • Or: reduction below “high” dose = 120 MED AND: “A responder also had to have, at worst, no increase in pain as a result of the intervention. Both aspects of improvement had to be maintained for at least three months post intervention. ” • OPIOID USE: no consistent assessment within the 5 studies. MED in only one, dose/week in another, vague in the other three. • All other outcomes: AEs, Mood, Physical Function, Pain inconsistently reported. Eccleston, C. , et al. (2017). "Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. " Cochrane Database of Systematic Reviews 11: Cd 010323.

Chronic Pain Opioid Reduction Cochrane Review, 2017 • My CONCLUSION: nothing really helpful here! • 50% reduction is a high bar, isn’t any reduction meaningful? • Studies should use IMMPACT guidelines for reporting outcomes in multiple domains • They note: “More work is needed to agree the best endpoints for treatments of medication reduction. Measures of the type of opioid and the median daily opioid dose in morphine equivalents consumed in a particular time period are critical to report. In addition, measures of patient-relevant outcomes such as mood, social functioning, and personal role functioning are also important to assess. ” Eccleston, C. , et al. (2017). "Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. " Cochrane Database of Systematic Reviews 11: Cd 010323.

Equally Revealing of Deficiencies: • Identified 61 studies. Quality: good = 3, fair = 13, poor = 51. • One of the “good” studies did not have opioid reduction as a primary objective • Limitation: Heterogeneous interventions and outcome measures; poorquality studies with uncontrolled designs. • Conclusion: “Very low quality evidence suggests that several types of interventions may be effective to reduce or discontinue LTOT and that pain, function, and quality of life may improve with opioid dose reduction. ” Frank, J. W. , et al. (2017). "Patient Outcomes in Dose Reduction or Discontinuation of Long-Term Opioid Therapy: A Systematic Review. " Annals of Internal Medicine 167(3): 181 -191

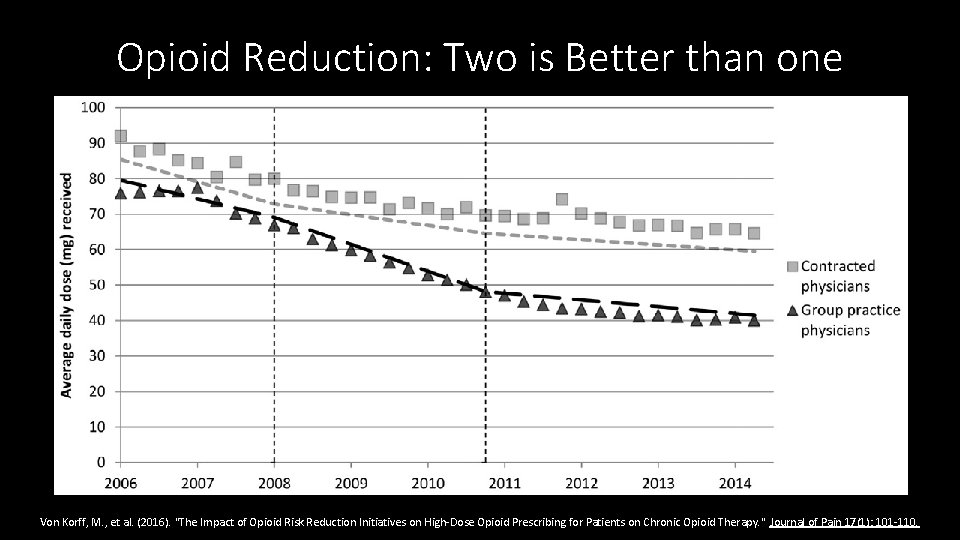
Education & Policies: A double dose is better • Washington state guidelines regarding limiting high dose opioids published 2007, law in 2010 • Group Health added initiatives/education for their providers, but not contracted providers. • Comparison of those exposed to just the state regs vs the double dose of intervention, 5, 552 COT patients (contracted providers) vs 16, 653 COT patients (group practice) • Study Measures: MED, > 120 MED, More than 20 excess days supply • Interrupted time series analyses • Reductions were substantially greater in the group practice setting that implemented additional initiatives to alter shared physician expectations regarding appropriate COT prescribing, compared with the contracted physicians’ patients. Von Korff, M. , et al. (2016). "The Impact of Opioid Risk Reduction Initiatives on High-Dose Opioid Prescribing for Patients on Chronic Opioid Therapy. " Journal of Pain 17(1): 101 -110.

Opioid Reduction: Two is Better than one Von Korff, M. , et al. (2016). "The Impact of Opioid Risk Reduction Initiatives on High-Dose Opioid Prescribing for Patients on Chronic Opioid Therapy. " Journal of Pain 17(1): 101 -110.

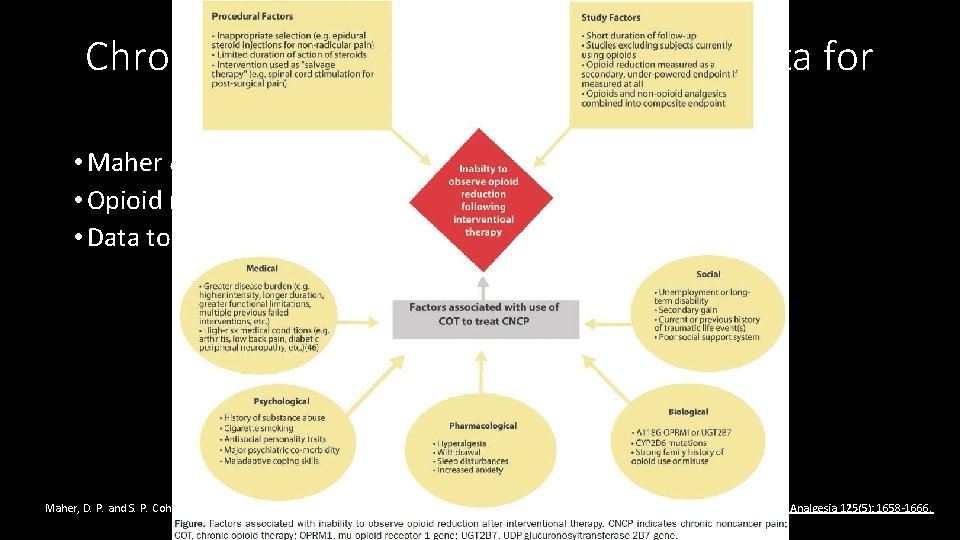
Chronic pain intervention, not much data for opioid reduction • Maher & Cohen review • Opioid reduction not a primary outcome • Data to support opioid reduction very limited Maher, D. P. and S. P. Cohen (2017). "Opioid Reduction Following Interventional Procedures for Chronic Pain: A Synthesis of the Evidence. " Anesthesia & Analgesia 125(5): 1658 -1666.

Conclusions ACUTE PAIN • Pain, satisfaction, function outcomes discussed elsewhere • Acute pain studies report many outcomes • Many opioid dosing outcomes can have meaning, those that extend beyond the immediate postoperative period are rarely reported CHRONIC PAIN • MED is the most common reported variable • High dose patients: using MED often reported as an additional outcome point • Many challenges

THE unanswered central question What opioid dose outcome(s) is/are sufficient to claim/support a meaningful “opioid-sparing” effect?

Thank You!
 Rass vs poss
Rass vs poss Opioid analjezikler
Opioid analjezikler Bromazepam umrechnungstabelle
Bromazepam umrechnungstabelle Opioid overdose
Opioid overdose Opioid overdose
Opioid overdose Opioid overdose
Opioid overdose Types of pain
Types of pain Mechanism of action of opioid analgesics
Mechanism of action of opioid analgesics Celeste martin
Celeste martin Opioid receptors location
Opioid receptors location Brian erickson md
Brian erickson md Equianalgesia
Equianalgesia Non-opioid
Non-opioid Kentucky opioid response effort
Kentucky opioid response effort Teaching and assessing grammar in the writing classroom
Teaching and assessing grammar in the writing classroom Ncbts and ppst difference
Ncbts and ppst difference Strengths and weaknesses in reading
Strengths and weaknesses in reading Assessing a new venture's financial strength and viability
Assessing a new venture's financial strength and viability Assessing leadership and measuring its effects
Assessing leadership and measuring its effects Module 4 topic 1 assessing and managing risk
Module 4 topic 1 assessing and managing risk Module 4 topic 1 assessing and managing risk
Module 4 topic 1 assessing and managing risk Assessing risk in sport regulatory bodies
Assessing risk in sport regulatory bodies Unit 18 assessing children's development support needs
Unit 18 assessing children's development support needs Task analysis in hrd
Task analysis in hrd Manual for assessing safety hardware
Manual for assessing safety hardware Cultural dynamics in assessing global markets
Cultural dynamics in assessing global markets Assessing cardiorespiratory fitness
Assessing cardiorespiratory fitness A nine box matrix requires assessing employees on ________.
A nine box matrix requires assessing employees on ________. Methodology for assessing procurement systems
Methodology for assessing procurement systems