OPIOID TASK FORCE Treating the Opioid Crisis Introduction



















- Slides: 29

OPIOID TASK FORCE Treating the Opioid Crisis: Introduction to Medication Assisted Treatment (MAT) Dr. Joy P. Alonzo, M. Engineering, Pharm. D Mental & Behavioral Pharmacotherapy Specialist Texas A&M Health Science Center Director of Spring Outreach Services

Learning Objectives Examine risk factors & clinical features of Opioid Use Disorder Assess FDA approved pharmacotherapy for OUD Differentiate between treatment strategies for OUD Discuss risk factors associated with MAT & prevention Determine the role of Harm Reduction in OUD OPIOID TASK FORCE

The Problem with Opioids: Different than Other Substances § Pain is subjective – no data to measure outcomes, patients expect to experience no pain, prescribers incentivized to provide opioids. § Changes in regimen cannot be done quickly due to physiologic responses and complexity of involved systems. § Used across various practice specialties, special populations. § Opioids do not have a maximum daily doses like most other medications. § Opioid Induced Hyperalgesia = ongoing or worsening pain. § Can’t get rid of prescription opioids, they have legitimate medical uses & no current credible substitute! (unlike other substances like cocaine/marijuana/meth) OPIOID TASK FORCE

The Opioid Crisis Contributing Factors § Incentivization of prescribers to provide opioid pain management: § TJC standards, MCO Star ratings, guideline recommendations § The inherent qualities of opioids can easily contribute to misuse. § These qualities include inducing feelings of euphoria and stress relief, as well as side effects such as tolerance and withdrawal. § Untreated underlying mood disorder like anxiety/high stress § Even when taken as prescribed, patients may still become addicted. § ALL OPIOIDS CAUSE: Tolerance/Dependence § Not all patients take opioids as prescribed. § High pain levels due to untreated illness § Illicit opioids (heroin, illicit fentanyl) are cheap and easy to get. OPIOID TASK FORCE

Opioid Use Disorder Risk Factors § Risk factors for opioid misuse: § Past or current substance use § Chronic Opioid Use § Untreated psychiatric disorders § History of alcohol and/or Benzo use § Youth and adolescents § Social or family environments that encourage misuse. § Comorbid psychiatric illness § Living in rural areas and having low income § History of Incarceration OPIOID TASK FORCE https: //www. cdc. gov/rxawareness/pdf/Overview-Rx-Awareness-Resources. pdf

Providing Relief from suffering for patients on the day you see them… MEDICATION ASSISTED TREATMENT OPIOID TASK FORCE

OUD MAT Treatment Goals • Always incorporate a patient’s goals when developing a treatment plan, which may include: • • Current interest in treatment Interest in abstinence based treatment versus MAT Work, community, justice supports Understand risk/benefits of approaches Agrees to clinic contract, safety precautions Adherence Financial resources Availability of wrap-around care OPIOID TASK FORCE

Harm Reduction … • Incorporates a spectrum of strategies including: safer techniques, managed use, and abstinence • Meets people “where they are” but doesn’t leave them there. • Applies evidence-based interventions to reduce negative consequences: • • • Medication Assisted Treatment Naloxone rescue kits Syringe exchange programs Pr. EP (Pre-exposure prophylaxis for HIV) Fentanyl test strips HEP C testing /HIV testing & education on prevention/treatment • Works to elicit ANY POSITIVE CHANGE based on individual patient need, circumstance, and readiness to change. OPIOID TASK FORCE

OUD MAT Treatment Goals • Objectives of MAT • A collection of Evidence-based treatments to: • • OPIOID TASK FORCE Decrease illicit opioid use Reduce transmission of Hepatitis C Reduce transmission of HIV Decrease criminal behavior Reduce sexual risk behaviors (e. g. , trading sex for money/drugs) Improve social functioning Retain people in treatment Decrease overdose and death

OUD Assessment • Physical Examination § § § Vitals Signs of injection Edema of extremities Jaundice Signs of: abscesses, cellulitis, endocarditis, tuberculosis § Signs of opioid withdrawal or intoxication • Lab tests § BMP/CBC, Viral Hep B/Hep C, HIV, TB § If appropriate, STDs, Pregnancy OPIOID TASK FORCE § Psychiatric Assessment § Severe, unstable, untreated illness § Psychoactive medication therapy § Substance Use § Opioid of choice, route, frequency, amount, duration § Other substance use § PMP history § Toxicology screen for substances § Substance Use Treatment § Past treatment types § Results of treatment § Reduction/cessation of use § Duration of cessation

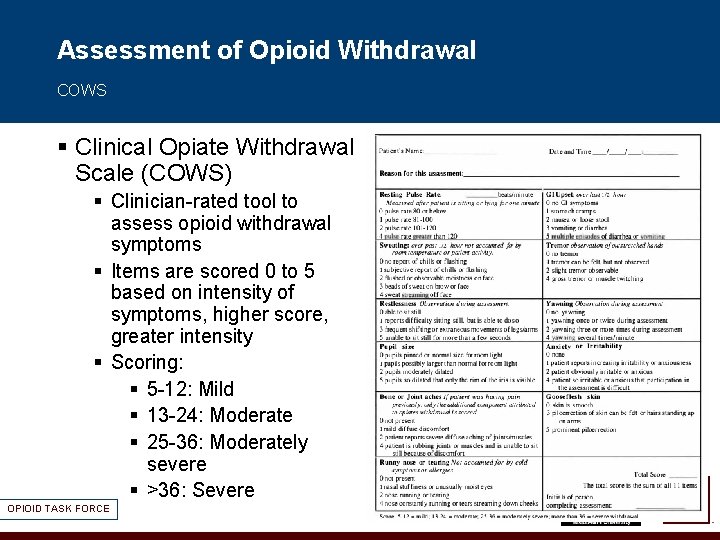
Assessment of Opioid Withdrawal COWS § Clinical Opiate Withdrawal Scale (COWS) § Clinician-rated tool to assess opioid withdrawal symptoms § Items are scored 0 to 5 based on intensity of symptoms, higher score, greater intensity § Scoring: § 5 -12: Mild § 13 -24: Moderate § 25 -36: Moderately severe § >36: Severe OPIOID TASK FORCE

Medications for Opioid Use Disorder • Long term maintenance therapy (18 mos or more) • 3 FDA approved medications : • Methadone • Buprenorphine • Naltrexone • Detox: is not treatment, management of withdrawal • Inpatient vs. outpatient • Strategies are colloquial, include a variety of medication management techniques, often feature titration of clonidine, clonazepam, symptomatic treatments OPIOID TASK FORCE

MAT : Place in the “toolbox” • One of the many tools in the “recovery toolbox”. • Reduce cravings which can help stabilize & strengthen coping capacity. • Increase periods of abstinence & instill a sense of self-efficacy. • Allows patients to focus on behavioral therapies. • Improve clinical outcomes for patients & reduce impact on families. OPIOID TASK FORCE

Methadone • Regulation: strict federal guidelines dictate eligibility for methadone treatment, prescribing circumstances, and access. • Benefit: prevents withdrawal symptoms, reduces cravings, reduces euphoria of subsequent opioid use, high efficacy in opioid use disorder AGONIST: long activation of receptor OPIOID TASK FORCE • Risk: possible overdose risk, misuse, hyperalgesia, cardiac arrhythmias, dependence

Methadone: Special Considerations • Synthetic, slow acting full mu-opioid receptor agonist • Half-life 24 -36 hours • Blocks euphoric effects of self administered opioids • Eliminates cravings for opioids • Used for treatment of substance use disorder since 1960’s • Only available through licensed Opioid Treatment Programs (OTP) • Dispensed @ OTP daily initially • Patients can progress to receiving “take-home doses” OPIOID TASK FORCE AGONIST: long activation of receptor

Buprenorphine Regulation: certified and specially trained clinician; patient limits in treatment (DEA X Waiver) , CIII, can be prescribed by in clinic, dispensed by community pharmacy Benefits: Detox and maintenance therapy, craving reduction, combined with naloxone to prevent misuse, good efficacy in opioid use disorder Several dosage forms including daily oral (tabs, films, SL), long-acting implant, long acting injectable. Some mixed with naloxone Risks: Must initiate brief withdrawal (4 -12 hours), misuse risk, street value due to withdrawal aid, dependence OPIOID TASK FORCE PARTIAL AGONIST: partial activation, partial blockade

Buprenorphine: Special Considerations • When prescribing buprenorphine to treat OUD outside of an OTP , physician must have a DATA 2000 Waiver, also called an “X-DEA number” EXCEPTION if using buprenorphine in hospital setting in accordance with the opioid withdrawal facility approved order set • DATA 2000 Waiver can be obtained by any physician by taking an 8 hour online course (DEA X Waiver) • Must complete buprenorphine training • https: //www. aaap. org/clinicians/education-training/mat-waiver-training/ • Allows you to treat 30 patients with buprenorphine in year 1, and 100 patients starting in year 2 • As of 8/2016, specific providers can treat up to 275 patients per year • In May of 2018, NPs and PAs can complete 24 hrs of training to obtain waiver OPIOID TASK FORCE

Buprenorphine Induction & Treatment • Must initiate withdrawal (4~12 hours since last opioid dose) • Induction achieved with 4 -8 mg of buprenorphine • Induction accomplished in a couple of days • Patients come to office weekly to start • 1 week supply of medication OPIOID TASK FORCE • Urine specimen @ each visit, clinic agreement • Psychosocial treatment required • Typically 3 rd party payer requirement • When stable, advance treatment • Give 2 week supply • If relapse, resume weekly visits

Buprenorphine Maintenance • Maintenance dose ~1224 mg per day • Patients can successfully be maintained at lower doses • Typically take oral dosage forms daily, but can take every other day (T 1/2=36 hours) • Concerns of risk of diversion at higher levels OPIOID TASK FORCE • Possible Adverse Effects: • • • Diaphoresis Constipation Headache Insomnia Nausea / Vomiting Hypotension Sexual dysfunction Seizures Hepatitis, hepatotoxicity

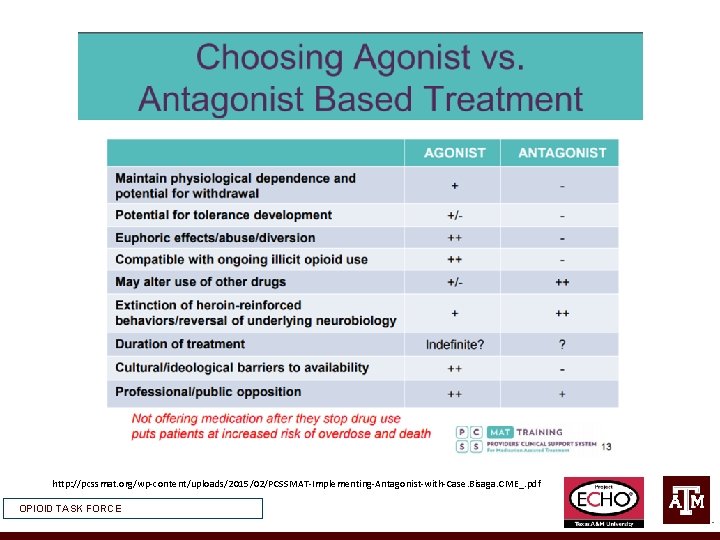
http: //pcssmat. org/wp-content/uploads/2015/02/PCSSMAT-Implementing-Antagonist-with-Case. Bisaga. CME_. pdf OPIOID TASK FORCE

Candidates for Naltrexone (Antagonist • Patients not able to be on agonist (buprenorphine or methadone) • High motivation for abstinence • Profession where treatment with agonist controversial • Patients successful on agonist but want to try abstinence • Failed prior treatment with agonist • Abstinent, but at risk for relapse • Patients for whom relapse would be disastrous • Patient with less severe form of disorder • Short history of use, lower level of use OPIOID TASK FORCE

Naltrexone • Limitations: requires completed withdrawal (714 days) from opioids (will precipitate withdrawal if taken with opioids in the system); requires highly motivated patient • Benefits: prevents opioid intoxication and dependence, reinforces abstinence, efficacy in opioid use disorder, no addiction potential, long acting injectable, drop out rate as high as 70 -80% due to withdrawal as necessity prior to initiation. 39% drop out with injectable. • Risks: may have increase risk of death from overdose due to decrease in tolerance with receptor blockade (depending upon dose of opioid used in relapse) OPIOID TASK FORCE ANTAGONIST: no activation, blocks opioids

Naltrexone Induction • No single best method but rather a set of approaches/tools that can be individualized to individual patient and the treatment team • Effective method will balance the degree of discomfort and the duration of treatment • Available as PO and long-acting injectable product • PO rarely used in treatment of OUD • Side effects: • Nausea/vomiting • Injection site reactions • Serious adverse effects: Hepatic injury @ high dose, suicidality • Contraindications/Monitoring • Elevated LFTs/Hepatic dysfunction • Cr. Cl < 50 ml/min • Recent opioid use (requires opioid wash out 7 -14 days) • Pain requiring opioids OPIOID TASK FORCE

Naltrexone Clinical Challenges Protracted Withdrawal: Naltrexone Flu • Patients who start naltrexone right after detoxification commonly experience “flu-like” signs and symptoms: • Somatic complaints: insomnia, GI distress, hyperalgesia, anergia • Anxiety, irritability, dysphoria, anhedonia • Severity may be lower if naltrexone is started 10 -14 days after completion of detoxification (but many relapse by then) • Partially alleviated with aggressive symptomatic treatment, • Insomnia (v. frequent, often severe): zolpidem, trazodone, quetiapine • GI distress: Ranitidine, PPIs • Anxiety/hyperarousal: clonazepam, clonidine • Most of these symptoms remit after 2 -4 weeks • True prolonged symptoms are rare and likely reflect additional psychopathology • Persistent/protracted withdrawal vs. acute effects of naltrexone • Negative mood and vegetative symptoms are significantly higher in participants who are receiving higher dose of naltrexone OPIOID TASK FORCE

Naltrexone Clinical Challenges • Testing the Blockade • 50% of patients “test” blockade same day of discharge, test 1 -3 times w/ low dose of opioid • Managing Relapse • Increased craving, use in week 3 -4, may need oral supplementation • Missing doses/injections: sign of relapse • Blockade 2 -3 days after stopping oral med, and 5 -6 weeks after injectable doses • May need re-stabilization/detox • Residential treatment/sober house • Transition to agonist OPIOID TASK FORCE

Naltrexone Safety Concerns: Overdose • Risk of overdose is significant if patient decides to stop taking naltrexone, stop attending treatment and resumes opioid use • Provide detailed description of risks (signed consent for treatment), continue discussing risks in patients who continue use: • “I understand that after I stop naltrexone I may be more sensitive to the effects of heroin and any other narcotics. The amount of heroin or narcotics I may have been using on a routine basis before I started naltrexone, might now cause overdose and death. I fully understand the nature and seriousness of this possible consequence. If I am not sure that I can avoid opiate use, I understand that I can be referred to alternative treatment programs, such as a methadone maintenance, which is an effective treatment for heroin dependence and has a reduced risk of fatal overdose. ” • Consider transition onto agonist to decrease risk of overdose if unable to comply with NTX • Fear of overdose applies to any completed detoxification or discontinuation of agonist maintenance. Naltrexone, especially longacting, actually PROTECTS against overdose OPIOID TASK FORCE

Co-Prescribe Naloxone for all OUD patients • Naloxone – short acting medication to remove opioid from opioid receptor, reverses opioid overdose • Co-Prescribing naloxone (NARCAN) is a best practice • Train Patient, care givers on use of naloxone to reverse overdose OPIOID TASK FORCE

Resources for the Clinician • Medication-Assisted Treatment of Opioid Use Disorder Pocket Guide • https: //store. samhsa. gov/system/files/sma 16 -4892 pg. pdf • Opioid Treatment Program Directory • https: //dpt 2. samhsa. gov/treatment/ • Buprenorphine Waiver Management (X Waiver or DATA 2000) • https: //www. samhsa. gov/programs-campaigns/medication-assistedtreatment/training-materials-resources/buprenorphine-waiver • Implementing Medication-Assisted Treatment for Opioid Use Disorder in Rural Primary Care: • https: //integrationacademy. ahrq. gov/sites/default/files/mat_for_oud_environ mental_scan_volume_1_1. pdf • Prescribe to Prevent • https: //prescribetoprevent. org/ OPIOID TASK FORCE

Questions or Comments jalonzo@tamhsc. edu